Deep brain stimulation (DBS) is a targeted therapeutic alternative for treatment-resistant depression that involves the bilateral placement of electrodes at specific neuroanatomical sites to deliver continuous stimulation from a subcutaneously implanted pulse generator (
7). We initially reported (
7) 6-month outcomes for six patients who received DBS to the subcallosal cingulate gyrus (Brodmann's area 25) for treatment-resistant depression, and we subsequently reported (
8) on 12-month outcomes in an expanded sample of 20 patients. In the latter group, the response rate was 60% (with response defined as a decrease of ≥50% in total score on the 17-item Hamilton Depression Rating Scale [HAM-D] [
9]) and the remission rate was 30% (with remission defined as a HAM-D score ≤7). Given the invasive and experimental nature of DBS for treatment-resistant depression, it is particularly important to obtain long-term effectiveness and safety data. Here we report on the extended follow-up of these 20 patients, with data from 3 to 6 years (mean=3.5 years) after DBS implantation.
Results
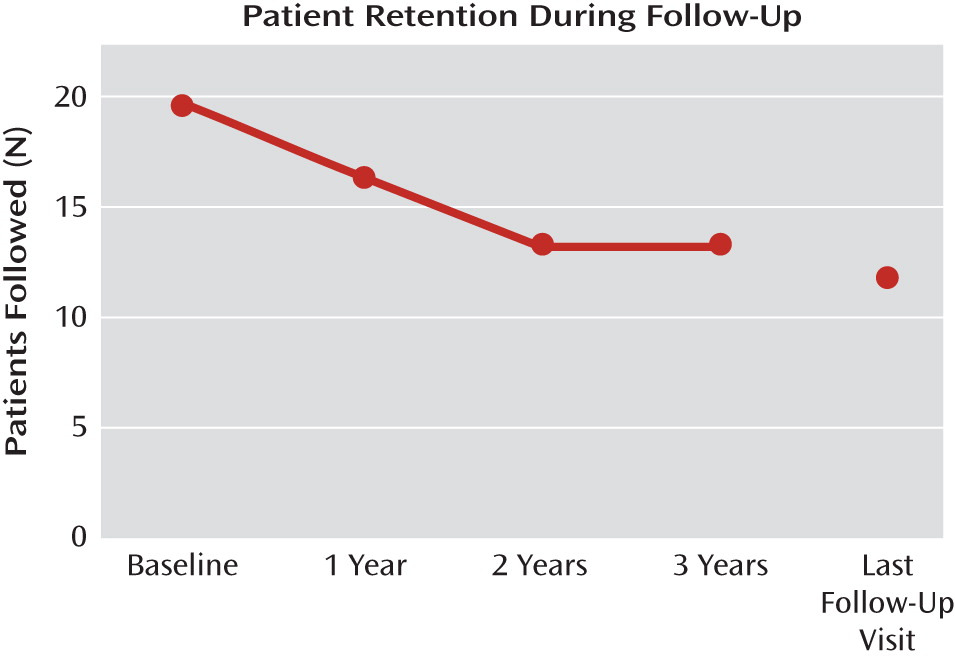
The mean duration of postsurgery follow-up for the cohort of 20 patients from DBS implantation to last follow-up visit was 42.1 months. The cumulative duration of follow-up was 841 months, or 70 patient-years.
Table 1 summarizes participants' demographic and clinical characteristics. Four of the 20 patients were unavailable for assessment at the 12-month follow-up: two had their DBS device explanted because of lack of efficacy, one left the country, and one was lost to follow-up.
During the second year, one patient died from an unrelated cancer and another patient requested explantation because of lack of efficacy, leaving 14 patients at the beginning of the third year. During this year, the patient who had previously left the country returned and reentered follow-up, and another patient died by suicide 35 months after DBS implantation. Of the 14 patients who completed year 3 of follow-up, five had not reached the end of year 4 by December 2009 (
Figure 1). Four patients were followed out to 6 years, and the death of one of these patients after 75 months was an unconfirmed suicide (see the Adverse Events section for details of suicides).
Clinical Effectiveness
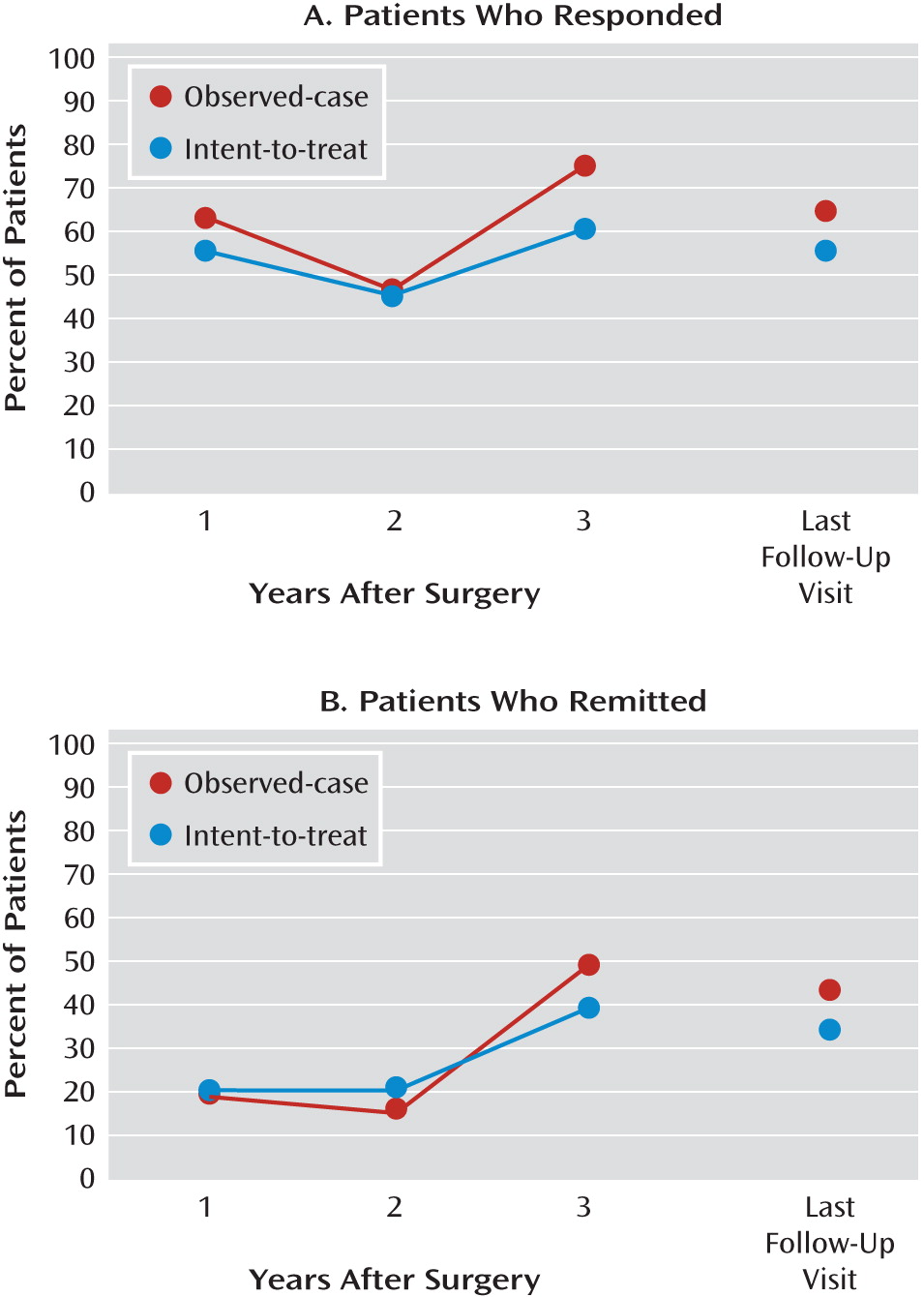
Percentages of patients who responded and remitted
In the observed-case analysis, the percentage of patients who responded was 62.5% after 1 year, 46.2% after 2 years, 75.0% after 3 years, and 64.3% at last follow-up visit. Using the intent-to-treat method, a similar pattern of response rates was noted, with 55% after 1 year, 45% after 2 years, 60% after 3 years, and 55% at last follow-up visit (
Figure 2). The majority of the responders at last follow-up visit (8 out of 11) had also been responders at year 1.
Remission rates over time also remained consistent: for the observed-case analysis, they were 18.8% after year 1, 15.4% after year 2, 50% after year 3, and 42.9% at last follow-up visit; and for the intent-to-treat sample, they were 20% after years 1 and 2, 40% after year 3, and 35% at last follow-up visit (
Figure 2).
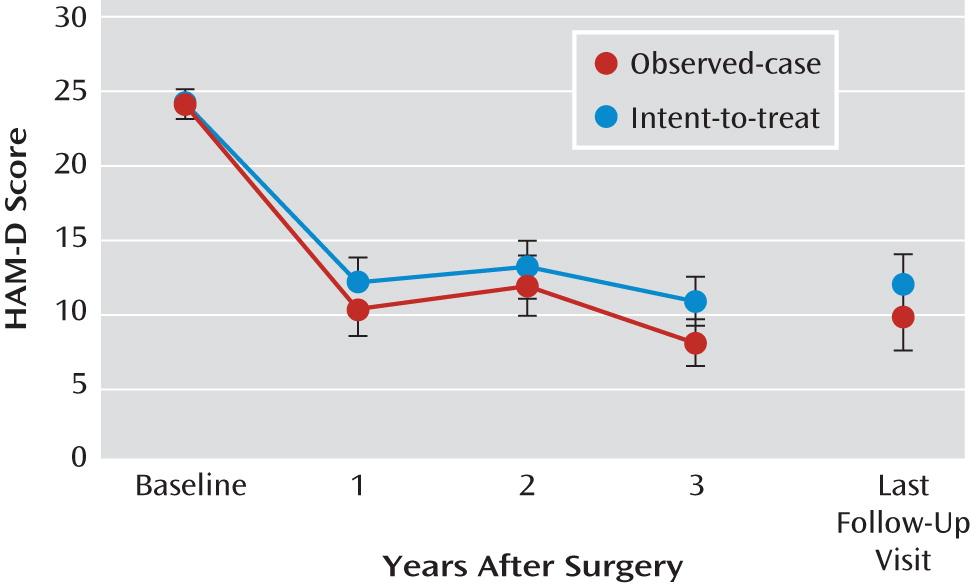
Decrease in HAM-D scores
HAM-D scores at last follow-up visit were significantly lower than at baseline (p<0.001), although they did not differ significantly from scores at years 1, 2, and 3. Across 3 years, HAM-D scores decreased significantly (F=34.5, df=3, 57, p<0.001). This decrease was significant from baseline to years 1, 2, and 3 (p<0.01), although HAM-D scores across years 1–3 did not demonstrate a statistically significant difference. The same outcomes were found using the observed-case data (
Figure 3).
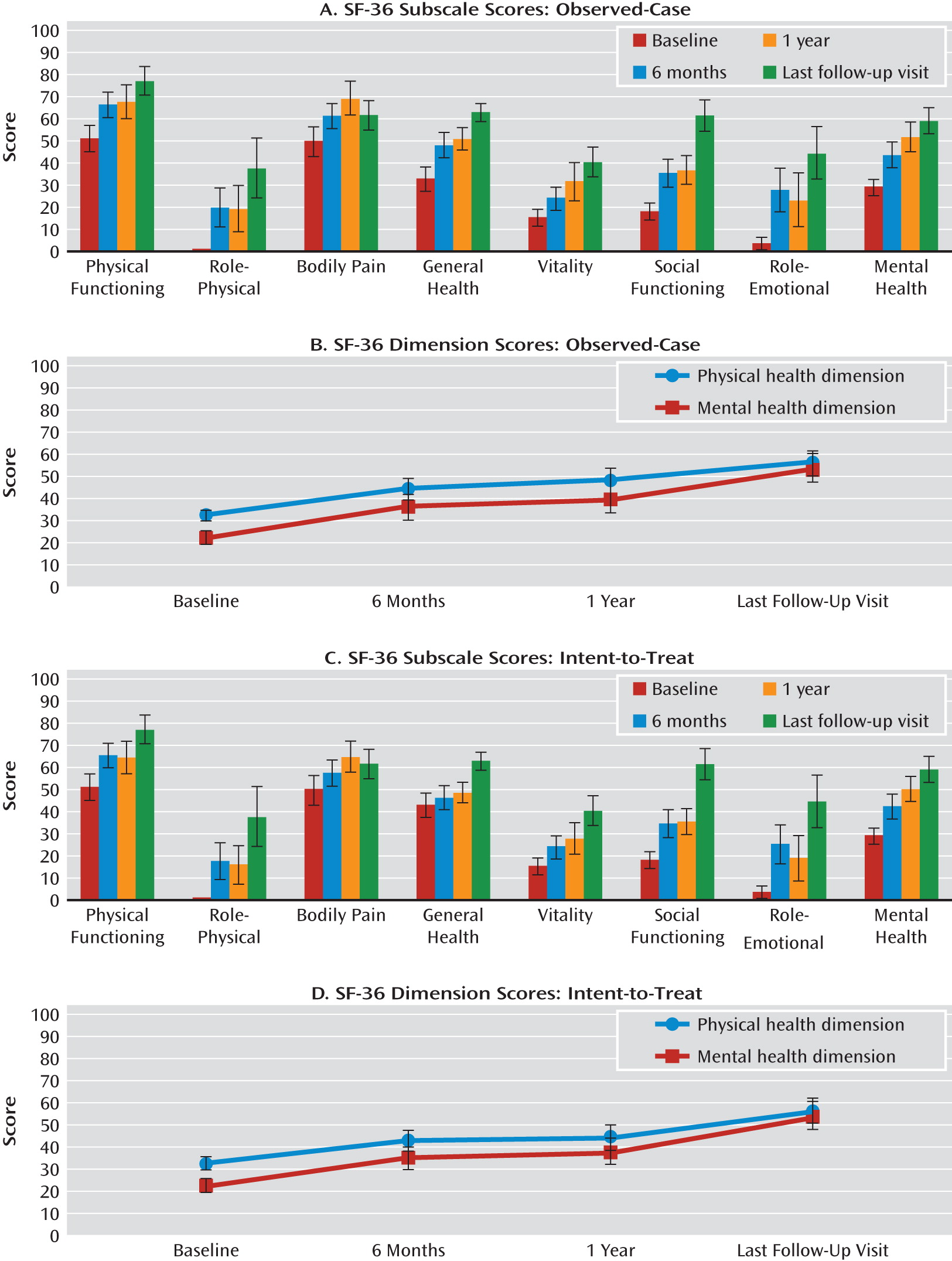
Functional Outcomes
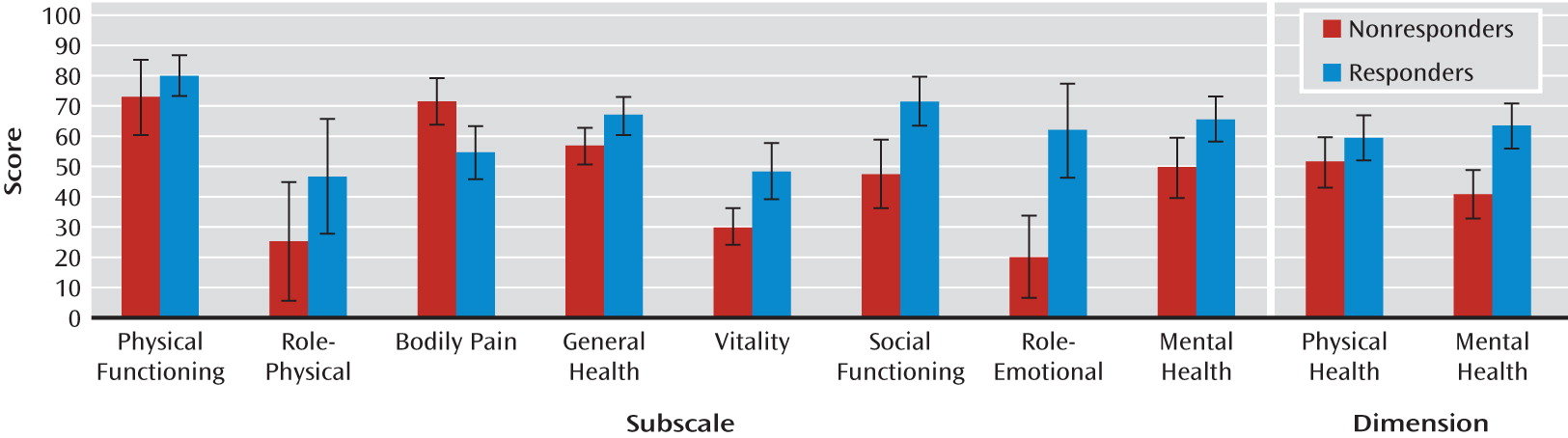
Figure 4 shows the mean SF-36 scores at baseline, month 6, year 1, and last follow-up visit for each of the eight subscales and for the physical and mental health dimensions. There was a significant effect of time on the social functioning (F=3.7, df=2, 24, p<0.05) and mental health (F=3.3, df=2, 24, p=0.05) subscales as well as on the physical health dimension (F=3.4, df=2, 24, p=0.05) (observed-case: baseline, N=19; month 6, N=18; year 1, N=13). There were no differences between scores at year 1 and at last follow-up visit (N=12).
In addition, there was a significant effect of time (intent-to-treat: baseline, N=19; month 6, N=20; year 1, N=16) on the physical functioning (F=3.8, df=2, 30, p<0.05) and mental health (F=4.0, df=2, 30, p<0.05) subscales, with significantly higher scores on both at month 6 compared with baseline (p<0.05). Scores were significantly higher at last follow-up visit (N=12) compared with year 1 on the social functioning (p<0.05), role-emotional (p<0.05), and general health (p<0.05) subscales as well as on the physical health dimension (p=0.05). There were no significant differences in SF-36 scores on any of the subscales or dimensions between responders and nonresponders at last follow-up visit (
Figure 5).
Work Status
The rate of employment at the time of DBS surgery was 10%. The rate increased to 50% by year 1 and onward. Three patients also began doing volunteer work, resulting in 65% of patients being engaged in work-related activities. Those who responded to treatment were more likely to return to work (90.9% of those who responded, compared with 33.3% of those who did not, p<0.05; odds ratio=20, 95% CI=1.7–238.6).
Adverse Events
Over the course of follow-up, eight patients were hospitalized for medical reasons on a total of 12 occasions. Half of these admissions were for psychiatric reasons (worsening depression, N=3; suicidal ideation, N=3), and the other half were for nonpsychiatric reasons (knee replacement, N=2; hemolytic uremic syndrome, N=1; pancreatitis, N=1; colon cancer, N=1; allergic drug interaction, N=1). Three patients were hospitalized more than once: one patient was hospitalized three times for worsening depression and suicidal ideation, and two patients were admitted twice for psychiatric and nonpsychiatric reasons.
The two patients in whom suicide was considered a probable cause of death accounted for four of the six psychiatric admissions. Both were distinguished from the two patients who made suicide attempts during postsurgery follow-up by prolonged hospitalizations early in the course of illness (patient 2 displayed aggressive behavior warranting the use of restraints during this extended admission) and a lack of functional integration into the workforce. The confirmed suicide victim had a family history of completed suicide in four first- or second-degree relatives (
Table 2). There was no evidence that any of the adverse events, including the deaths, were due to DBS device failure or changes in stimulation parameters. There was also no evidence in the full sample of 20 patients that the suicide item of the HAM-D increased in severity independently of total score.
Device and Medication Considerations
As previously reported (
7), three of the first six patients had hardware infections in the first 3 months after surgery. This complication was related to technical factors, including externalization of electrodes. The hardware was removed after 4 months and 6 months in two cases, respectively, and was not reinserted. In the third patient, the hardware was replaced without complications and with continuing clinical benefit. Subsequent patients had the electrodes and internal pulse generators implanted in a single session. No device-related adverse events occurred beyond those previously reported up to 1 year (
8). Eight battery replacement surgeries were required during follow-up (mean time to battery replacement, 43.3 months [SD=19.8]). One patient required battery replacements on two occasions, at 40 months and at 69 months.
In the course of routine follow-up of these patients, serendipitous discovery of battery depletion was noted. Clinically, it was correlated with a decline in mood over the previous 4–6 weeks. After battery replacement, improvements typically occurred within 2–4 weeks.
DBS Stimulation Parameters
Data on DBS stimulation parameters were available for 17 of the 20 patients. Monopolar contacts were used in all patients. By convention, the nomenclature for denoting the left quadripolar electrode contacts is 0–3, and for the right, 4–7 (Medtronic 3387, Medtronic, Inc., Minneapolis). Larger numbers refer to a more dorsal position of the contacts on the electrode (i.e., 3/7 is the most dorsal contact pair on the electrode, and 0/4 the most ventral). The 1/5 and 2/6 electrode contact pairs in this study were implanted in the subcallosal cingulate gyrus white matter bundle (
11).
The majority of the patients received bilateral stimulation of symmetrical pairs of contacts (N=13), while two were receiving unilateral stimulation at last follow-up visit (in one patient, the left contact 1 was on and the right was off, and in the other the left contact 2 was on and the right was off). Two patients received stimulation of two pairs of symmetrical contacts (1/5 and 2/6). Of those who received symmetrical bilateral stimulation (N=13), the 1/5 pair of contacts was used in 10 patients, and the 2/6 pair was used in three patients. The likelihood of achieving long-term response was not increased by using the 1/5 contacts (odds ratio=0.4, 95% CI=0.05–3.3).
The average voltage used for the entire group was 4.3 V (SD=1.7). There was no statistically significant difference between the voltage received by patients who were responders at the last follow-up visit (mean=4.3 V, SD=1.5, range=2.5–8.0) and those who were not (mean=4.4 V, SD=1.2, range=2.5–5.5). The mean frequency for the group was 124.7 Hz (SD=21.8), and the mean pulse width was 70.6 μsec (SD=14.8). In summary, there were no differences in the anatomical placement of the electrode or the DBS stimulation parameters between patients who responded and those who did not.
Medication Status
In general, patients required less medication after DBS implantation. By last follow-up visit, nine patients had decreased the number of antidepressants taken, while only one patient commenced antidepressant therapy. Among the 10 patients who remained on the same number of antidepressants, the dosage was decreased in four and increased in one. Five of the 14 patients receiving an atypical antipsychotic discontinued this medication, while four had a dosage increase. Four of the 18 patients reduced the number of benzodiazepines or hypnotics from baseline to last follow-up visit, and one patient started a new benzodiazepine.
Discussion
This study, which represents over 70 patient-years of assessment, is the longest follow-up report to date on the largest cohort of patients with treatment-resistant depression who have received DBS. Response rates of 60% at 3 years and 55% at last follow-up visit are comparable to those of our previous reports at 6 months (
7) and 1 year (
8). Notably, more than one-third of patients were in remission at year 3 and at last follow-up visit. Although these results are derived from an open-label DBS study with no control group, the remission rates compare favorably to rates of less than 8% reported in a cohort of less severely ill patients with treatment-resistant depression receiving standard antidepressant treatments under naturalistic conditions (
12). The consistent response rates seen in our initial cohort of DBS patients suggest that the delayed but progressive improvement in depressive symptoms tends to be maintained over several years.
DBS for psychiatric illness is a nascent area of investigation, and the optimal neuroanatomical target(s) and stimulation parameters have yet to be determined. Other investigators have used different neuroanatomical targets for stimulation, including the ventral capsule/ventral striatum (
13) and the nucleus accumbens (
14). Malone and colleagues (
13) reported response rates of 40% and 53.3%, respectively, at 6 months and last visit for 15 patients with treatment-resistant depression during a variable follow-up period ranging from 6 months to 4 years. Similarly, Bewernick and colleagues (
14) reported a response rate of 50% at 12 months for 10 patients with treatment-resistant depression. The response rates in these studies are comparable to the short- and long-term outcomes reported here. This raises the possibility that stimulation at multiple target sites may influence activity at different points of the neurocircuitry subserving emotion regulation (
15,
16). To date, there have been no head-to-head clinical comparisons of the candidate DBS targets for treatment-resistant depression, so it remains unknown whether the putative targets may differentially improve some depressive symptom clusters.
Treatment-resistant depression has been associated with poor clinical outcomes (
17) and impaired long-term social functioning (
18). We previously reported no negative neuropsychological effects of subcallosal cingulate gyrus DBS in the first six patients of this series up to 12 months after DBS implantation (
19). In the present study, functional outcomes improved after implantation, as indicated by return to work status and self-reported quality of life. While improvements in physical functioning were observed by 6 months, there was a delay before benefits became apparent on the social functioning, role-emotional, and general health subscales of the SF-36, and improvements continued over time and were statistically superior at last follow-up visit compared with year 1.
From a public health point of view, both the duration and the severity of illness are important determinants of the disease burden of major depression (
20). Treatment-resistant depression, which is characterized by longer duration and greater illness severity, represents a large proportion of the societal burden attributable to major depression (
21). Although the cost-effectiveness of DBS for treatment-resistant depression has not been established, the findings that over 50% of patients returned to gainful employment, required fewer medications, and experienced improvements in quality of life suggest long-term direct and indirect cost savings associated with DBS.
Adverse events are a common reason for treatment discontinuation in major depression (
22). There were no DBS device-related issues in the long-term follow-up of this cohort, and the voltage parameters were generally well tolerated. The mean voltage used for the entire group was 4.3 V, comparable to the range reported for DBS to the nucleus accumbens (
14) but different from the mean voltage of 6.7 V reported for DBS to the ventral capsule/ventral striatum (
13). The average time to battery replacement in the present study was 43.3 months, compared with 10.6 months reported by Malone and colleagues (
13). Most but not all studies (
23) have reported that lower voltages are associated with better clinical results. However, in an extension of the preliminary findings by our group (
11,
24), long-term antidepressant outcome was not predicted by stimulation parameters or regional variation in the placement of electrodes in the subcallosal cingulate gyrus.
The death of three of the original cohort of 20 patients emphasizes the high rates of mortality associated with treatment-resistant depression. One patient died from previously undiagnosed colon cancer, which was unrelated to DBS. Despite achieving remission for extended periods during the study, suicide was a likely cause of death for the other two patients (see
Table 2), although an accidental overdose cannot be ruled out in one of the patients. This patient was being monitored regularly by one of the treating psychiatrists (P.G.), who saw her 2 weeks before her death and observed no change from persistent passive suicidal ideation (her HAM-D suicide item score was 2). The third patient who died was in the care of a community psychiatrist for the 4 months before death but was seen by one of the psychiatry team (P.G.), who confirmed that there was no stimulator or battery malfunction. Although the patient had previous periods of sustained remission, she relapsed and was admitted with active suicidal intent (her HAM-D suicide item score was 3), which remitted after a course of ECT 3 months before her death.
The long-term outcome of depressed patients is characterized by high rates of medical comorbidity and mortality from both suicide and medical illness (
25,
26), with all-cause mortality rates in treatment-resistant depression estimated in two studies to be 13% over 4–8 years (
27) and 32% over 7 years (
28). The rate of suicide in depressed patients is frequently cited as 15% (
29), based on a follow-up study of severely ill hospitalized patients with a diagnosis of “melancholic depression.” Subsequent reevaluations suggest that approximately 2% of outpatients and 6%–15% of inpatients die by suicide (
30,
31). Given that this was a small open-label trial, it is difficult to determine whether the death by probable suicide of two patients (10%) is in excess of the expected mortality over a 3–6 year period. Furthermore, few antidepressant trials involving outpatients with treatment-resistant depression have provided data on suicide rates beyond 12 weeks.
In addition to hospitalization, risk factors for suicide include a history of previous suicide attempts, a family history of completed suicide, impulsivity, aggressiveness, and high levels of anxiety (
32–
34). In the present study, the two patients who committed suicide were among the most frequently hospitalized after DBS implantation, and one patient had a strong family history of suicide. Thus, previous psychiatric admissions and suicide attempts, despite concurrent DBS and psychiatric management, may be indicative of high suicide risk, and patients with these risk factors require more monitoring, even during remission or response.
Considering the limited number of trials of DBS in treatment-resistant depression, data on suicide in DBS patients are mainly derived from studies of patients with Parkinson's disease and essential tremor receiving DBS to motor nuclei of the thalamus or the subthalamic nucleus (
35). In longitudinal cohort studies of DBS for movement disorders, 4.3% of patients committed suicide, with an average time to completed suicide of 3 years after surgery (
35–
37). Completed suicides have also been reported in small open-label studies of DBS for other psychiatric conditions: nucleus accumbens DBS for obsessive-compulsive disorder (
38) and for treatment-resistant depression (
14). As in our study, the deaths in these studies did not appear to be related to DBS device malfunction or recent changes in stimulation parameters and were comparable to mortality rates reported in naturalistic studies of patients with treatment-resistant depression (
27,
28).
There are considerable limitations to this study. First, it was an open-label trial, which limits our ability to draw conclusions about the efficacy of DBS. Although it is possible that the symptom improvements seen were due to placebo effects or the nonspecific aspects of psychiatric care, sustained antidepressant response for longer than 3 years in a cohort of patients with treatment-resistant depression is inconsistent with a placebo response, particularly when battery failure was associated with return of symptoms. However, there is a need for double-blind sham-controlled studies to determine whether DBS is an efficacious antidepressant therapy. Second, the patients in this study suffered from nonpsychotic unipolar major depression, and it is unclear whether these results will generalize to patients with other subtypes of major depression or bipolar disorder. Third, only clinical assessments were carried out during the long-term follow-up of these patients. The lack of biological data and the small sample size limit analyses of biological mediators and moderators.
In summary, the rates of antidepressant response observed over 3 years of DBS are consistent with those previously reported (
7,
8). In addition to confirming the sustained effect of DBS on reducing depressive symptoms, this study presents additional findings on functional outcomes. More than half of patients returned to work, and improvements in quality of life beyond 1 year after DBS implantation suggest that there are both short-term and long-term benefits associated with DBS to the subcallosal cingulate gyrus for treatment-resistant depression. The death of two patients by suspected suicide suggests use of caution and reinforces the need for long-term psychiatric management, including psychosocial and pharmacologic therapies, in combination with DBS.