The primary model of the neurobiology of late-life depression is the vascular depression hypothesis (
1). The vascular depression model posits that vascular changes, primarily ischemic changes in the white matter, are a significant factor in the pathogenesis of late-life depression. This model has been supported by several neuroimaging studies that have demonstrated higher white matter hyperintensity (WMH) ratings in patients with late-life depression relative to nondepressed elderly comparison subjects (
2,
3). Another study has shown that indicators of the vascular depression phenotype (e.g., WMHs, executive function, and age at onset) are significant predictors of treatment response (
4). Several studies have suggested that the localization of WMHs in late-life depression may be specific to the frontal and subcortical regions (
5–
7), thus disrupting critical frontal-subcortical affect-regulating circuits.
Over the past decade, functional imaging, primarily positron emission tomography (PET) and functional MRI (fMRI), has been used to develop and test models of the functional neuroanatomy of depression (
8–
10). These studies have primarily used participants with midlife major depression and have consistently observed rostral and ventral brain hyperactivation in the depressed patients relative to nondepressed comparison subjects. The hyperactivation has been observed at rest in studies of cerebral metabolism ([
18F]fluorodeoxyglucose [FDG] PET [
9]) and also with blood-oxygen-level-dependent (BOLD) fMRI in response to affective tasks (
11). The particular fMRI tasks have varied, but a common approach is to have participants view faces that have emotional expressions. This task seems to be particularly effective at engaging the anterior and ventral structures, which include limbic regions such as the amygdala, the rostral cingulate, the medial prefrontal cortex, and the anterior insula. Hariri et al. (
12) reported that this emotional faces task is particularly effective at eliciting BOLD fMRI activity in the amygdala and in ventral limbic regions. Studies of midlife depression have also identified hypoactivation in the dorsal structures associated with cognitive control, including the dorsal anterior cingulate and the dorsolateral prefrontal cortex. According to the functional neuroanatomical models of depression (
10), depression can be described as altered patterns of neural regulation between the dorsal-cognitive and ventral-affective components of an affect regulation system.
This functional neuroanatomical model of depression is further supported by treatment studies of midlife depression that have shown that ventral hyperactivity responds to treatment. In a study of 11 depressed patients using an emotional faces task, the successful treatment of depression was correlated with a decrease in amygdala activity from pretreatment to posttreatment assessments (
4). Other studies have reported similar findings in the subgenual cingulate (
9). This greater activity in pretreatment midlife depression has served as the basis of innovative studies examining treatment-resistant depression (
13).
Functional imaging studies of late-life depression have replicated some aspects of the pattern observed in midlife depression. Some studies of late-life depression, but not all (
14), reported decreased dorsal cognitive activation (
15,
16) and greater ventral limbic activation (
15). A relatively small number of functional imaging studies of late-life depression, with relatively small sample sizes (e.g., N=13 per group [
16]), have been conducted compared to the number of larger studies reporting structural imaging findings in late-life depression (
4). The structural imaging studies have shown a significant correlation between late-life depression and white matter ischemic changes, which are visualized as WMH lesions on MRI (
6).
Our study relates the white matter structural changes in late-life depression to the functional models of depression that have been primarily developed through studies of midlife depression. In particular, are the white matter lesions of late-life depression associated with the same ventral limbic hyperactivity that is observed in midlife depression? Our study used a standard fMRI task (the faces and shapes task [
17]) that has been shown to activate the expected limbic structures (
18). WMH burden was assessed using an automated segmentation of T
2-weighted fluid attenuated inversion recovery (FLAIR) images. In this study we focused on overall global WMH burden rather than using regional WMH measures from the various regions and white matter tracts implicated in affective processing. The choice of whether to use global or regional WMH measures depends on whether the hyperintensities are viewed as a summary biomarker of overall global white matter disease (i.e., a global biomarker) or whether the WMHs are viewed as an indicator of localized white matter damage to the tracts intersected by WMHs (i.e., localized WMHs) instead. Evidence supports both the global biomarker view and the localized WMHs view (
6,
19). Our choice of using global WMHs as our primary marker in this study was motivated by the global biomarker view and our intent to limit the number of independent variables.
Method
Participants
We recruited patients with nonpsychotic, unipolar major depressive disorder from ongoing open-label treatment studies of late-life major depression (
20,
21) at the University of Pittsburgh's Advanced Center for Interventions and Services Research for the Study of Late-Life Mood Disorders. Depressed patients had an fMRI scan before starting antidepressant medication, and they were asked to participate in another scan 12–16 weeks after starting treatment. (Analyses of the follow-up scans will be presented in a separate report.) Nondepressed elderly comparison subjects were recruited from the community and from the healthy volunteer registry of the University of Pittsburgh Alzheimer Disease Research Center. After receiving a complete description of the study, each participant provided written informed consent. All participants were paid $50 to take part in the study.
All participants were assessed with the Structured Clinical Interview for DSM-IV (
22). Other than major depressive disorder and anxiety disorders, all axis I disorders were used as exclusion criteria. Other exclusion criteria were a history of stroke or significant head injury, Alzheimer's disease, Parkinson's disease, or Huntington's disease. We chose to include participants with anxiety disorders because of the high prevalence (48%) of anxiety disorders in individuals with late-life depression (
23). Individuals were excluded if they had taken psychotropic medications during the 2 weeks before imaging, but other medications were acceptable because their use is common in the elderly population.
Procedures
Faces and shapes affective reactivity task.
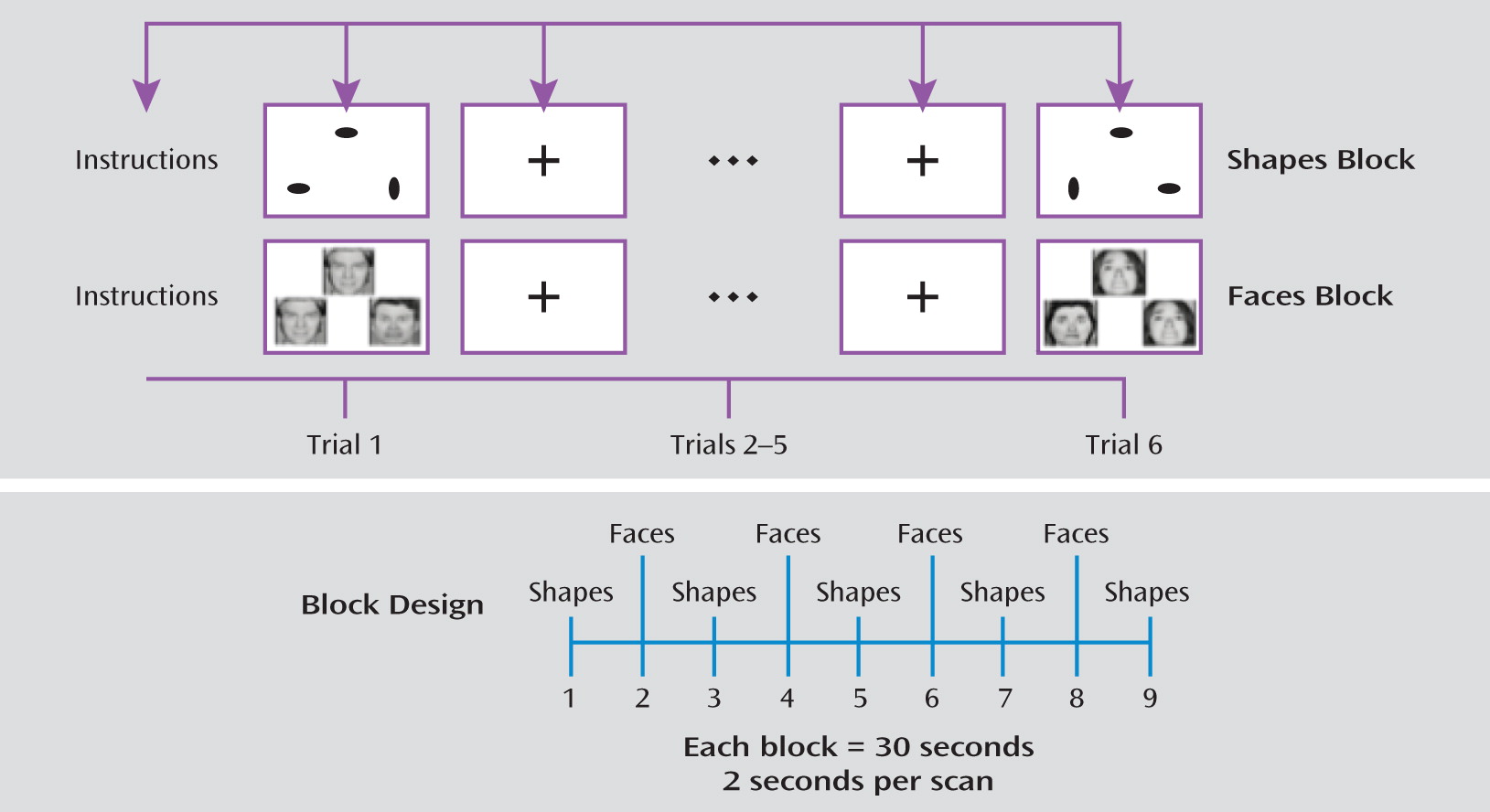
The faces and shapes fMRI task (illustrated in
Figure 1) has been used extensively to explore the neural circuitry of affective reactivity (
17). One study observed that subjects engage in spontaneous emotional regulation when exposed to this particular task (
24). In this task, participants are required to select one of two facial expressions (either angry or afraid) that matches a simultaneously presented target expression. As a control task, the participants match one of two geometric shapes with a simultaneously presented target shape. The task involves five blocks of the shape-matching task alternating with four blocks of the face-matching task. Each block lasts 30 seconds (six trials that last 5 seconds each). On the faces trials, 12 different images derived from a standard set of pictures of facial affect are used, six per block and three of each gender (
25). Stimulus presentation and response recording was controlled using the E-Prime software package (Psychology Software Tools, Inc., Pittsburgh, 2002).
Data acquisition.
Imaging data were collected with a 3-T Siemens Trio TIM scanner located in the MR Research Center at the University of Pittsburgh. For functional image alignment we used a T2-weighted sequence (TR=3,000 msec, TE=101 msec, in-plane resolution=1 mm×1 mm, 3-mm slice thickness, 48 slices). To assess for small vessel ischemic changes, we used a T2-weighted FLAIR sequence (TR=9,002 msec, TE=56 msec [effective]; TI=2,200 msec, number of excitations=1) using an interleaved acquisition (48 slices, 3-mm slice thickness, no gap). T2*-weighted BOLD acquisition was done using a gradient-echo echo planar imaging sequence: TR=2,000, TE=32, matrix=128×128×29, voxel size=2×2×3 mm3, oblique axial acquisition (parallel to anterior commissure-posterior commissure line), integrated parallel acquisition techniques=2. The most inferior functional scan was below the most inferior aspect of the temporal lobes.
Imaging analysis.
We used an automated WMH localization and segmentation method to compute the normalized WMH volumes (
7). For each participant, the calculated WMH volume was normalized with the overall brain volume. The automated WMH segmentation method (
7) is an iterative algorithm that involves an automated selection of “seeds” of possible WMH lesions and fuzzy connectedness, which clusters voxels based on their adjacency and affinity,to segment WMH lesions around the seeds (
26). The fully automated WMH segmentation system was implemented in C++ and ITK. (The WMH matter extraction algorithm is available at
http://www.gpn.pitt.edu.)
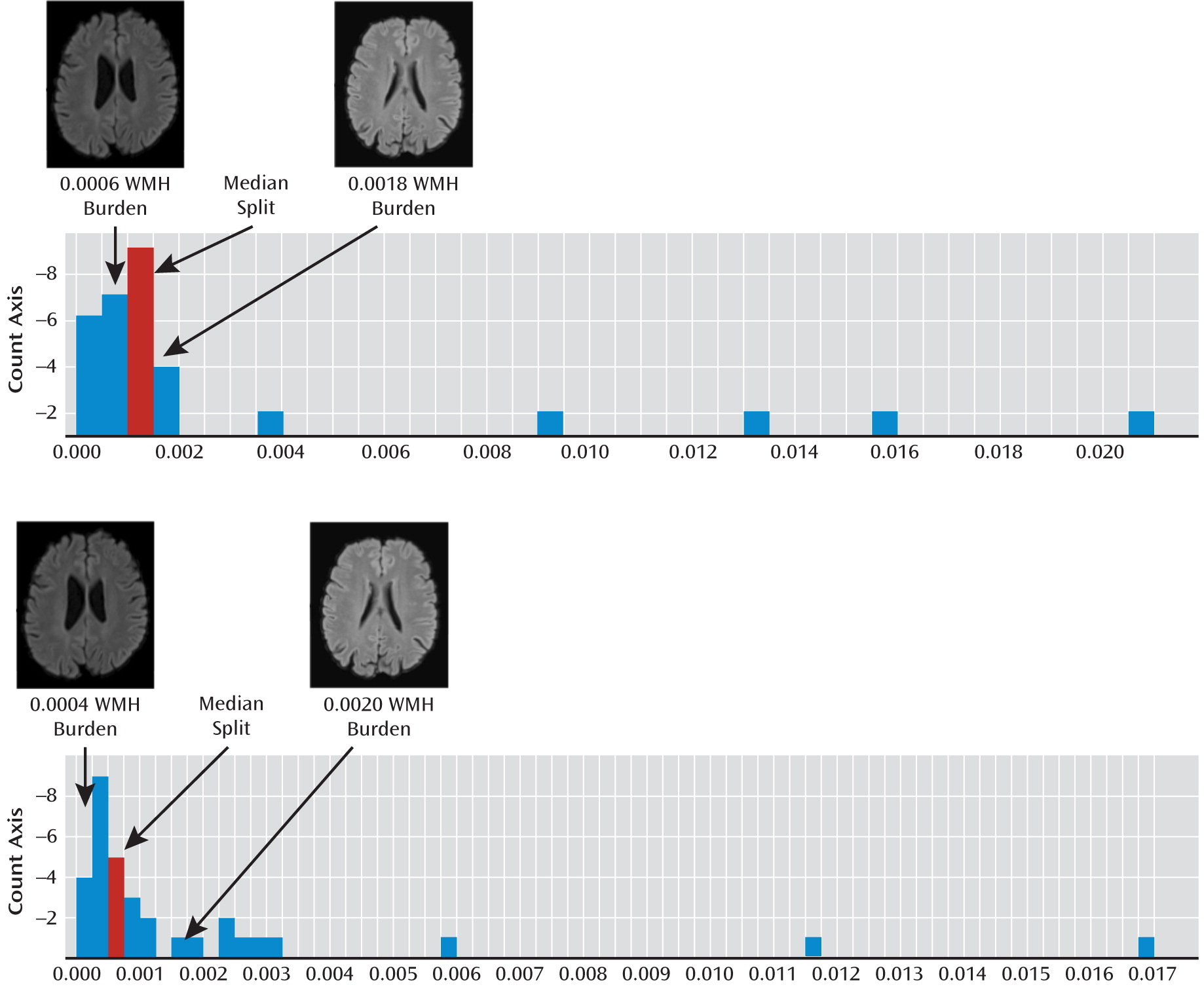
To assess the relationship of WMHs on the fMRI BOLD response pattern in late-life depression, we first identified the median normalized WMH volume among the late-life depression patients. This split was used to define a group with a low WMH burden (WMH burden < the median) and a group with high WMH burden (WMH burden ≥ the median) (
Figure 2).
fMRI Preprocessing and Group Analysis
Functional imaging data were analyzed with SPM5 (Wellcome Department of Imaging Neuroscience, London, 2007) implemented in MatLab (MathWorks, Natick, Mass.). For each participant, all echo planar imaging volumes were realigned to the first echo planar imaging volume, and a mean image of the realigned volumes was created. The realigned images were coregistered to the anatomical T
1-weighted image. To normalize the anatomical image as well as the echo planar images to a standard SPM reference system, the following procedure was applied. First, the anatomical image as well as a representative template image (from the Montreal Neurological Institute) was segmented into gray matter, white matter, and CSF. Then the anatomical gray matter image was normalized to the gray matter of the Montreal Neurological Institute brain. Subsequently, the derived normalization parameters were applied to the echo planar images, which were resampled to a voxel size of 2 mm×2 mm×3 mm and smoothed with a 10-mm wide Gaussian kernel to account for greater morphologic variability in an elderly sample (
27). All statistical analyses were performed in the context of the general linear model (
28).
Movement parameters derived from realignment were assessed, and no participant with excessive motion (>4 mm or >4°) was identified. We also added movement as a covariate of no interest to correct for confounding effects induced by head movement. The primary contrast of interest (faces > shape condition) was first estimated for each participant individually (first-level analysis) and then used in a second-level random-effects analysis to assess the group-level effects.
The fMRI analysis was performed on 60 participants. The second-level analysis initially included all of them in order to identify the main effect of the task. Second-level analyses were also done between groups (depressed patients relative to nondepressed comparison subjects) to identify the effect of depression, and in the depressed and comparison groups to identify the effect of WMHs. High and low WMH burden was designated by a median split of normalized WMH volume.
The second-level analysis over all participants was performed using a one-sample two-tailed t test. The between-group analyses were modeled using two-sample two-tailed t tests. In each group, the mean group maps were estimated using the difference between BOLD signal during faces and comparison conditions. We tested between-group differences in the BOLD signal during the task, and group differences were measured at each voxel and restricted to the gray matter. We set a t value threshold at a minimal voxel entry value of t>2.6618 (df=59) for main effects, t>2.6633 (df=58) for depressed patients relative to comparison subjects, t>2.7564 (df=29) for high relative to low WMH burden in the depressed group, and t>2.7874 (df=25) for high relative to low WMH burden in the comparison group (p<0.005, uncorrected). We then corrected for multiple comparisons using the family-wise error rate as implemented in SPM5, using a small-volume correction for the a priori regions of interest, which included the amygdala, the rostral cingulate, and the insula.
Results
We analyzed MR data from 60 right-handed participants: 27 nondepressed elderly comparison subjects and 33 patients with late-life depression. The participants' demographic characteristics are summarized in
Table 1.
Most of the depressed patients (21/33) had late-onset late-life depression, with their first episode of depression starting after age 50. Thirteen of the late-life depression patients and none of the comparison subjects met the criteria for an anxiety disorder. In almost all cases (85%), the anxiety disorder was generalized anxiety disorder, but there were also individual cases of specific phobia, posttraumatic stress disorder, social phobia, and anxiety disorder not otherwise specified.
Chronic elderly comorbidities such as hypertension and thyroid conditions were present in both the healthy comparison and depression groups. Although detailed medication records were available for only nine of the comparison subjects observed for these analyses, rates of such comorbidities were similar in both groups. Fifty-five percent of nondepressed comparison subjects were hypertensive, compared with 60% of depressed patients, and 11% of comparison subjects were using thyroid agents, compared with 18% of patients. None of the comparison subjects were taking psychoactive drugs at the time of the scan.
The mean Mini-Mental State Examination (MMSE;
29) score for the comparison subjects and the depressed patients was not significantly different. MMSE scores for two participants were unavailable.
White Matter Hyperintensity Assessments
Normalized WMH volumes were similar in the depressed and nondepressed groups, and no significant difference was observed in overall WMH volume ratings between the groups (t=1.061, df=58). Although previous studies, including studies from our research group, have observed higher WMH burden in late-life depression patients relative to comparison subjects (
7), other studies observed similar WMH burden between the groups (
5). It appears that the difference is partly related to the degree to which the patients and the healthy comparison subjects are matched in terms of cerebrovascular risk factors (
5). Our comparison subjects were recruited from the volunteer (nondemented) registry of the University of Pittsburgh Alzheimer Disease Research Center, which includes individuals with age-related chronic conditions, including high blood pressure.
A primary aim of this study was to evaluate the relation of WMH burden to functional (BOLD) response in late-life depression patients. These patients showed the expected variability in WMH burden.
Figure 2 shows the histogram of normalized WMH burden in the late-life depression group along with the median split that was used to divide the group into low and high WMH burden. To illustrate the degree of WMH burden in these two groups, T
2-weighted FLAIR images from a subject in the middle of the low-WMH-burden group and a subject in the middle of the high-WMH-burden group for both healthy comparison subjects and late-life depression patients are shown. No significant differences between high- and low-WMH burden participants were observed in demographic characteristics, cognition, or depression severity. Additionally, age at the time of depression onset (which has previously been associated with WMH burden [
5]) was also compared with WMH burden, and no significant association was observed. The fact that we did not find a significant correlation of WMH burden with age at onset in this sample of 33 elderly depressed patients may have been because of reduced power in looking at the subsample of the study (i.e., only depressed).
The Main Effect of Task (Faces Compared With Shapes)
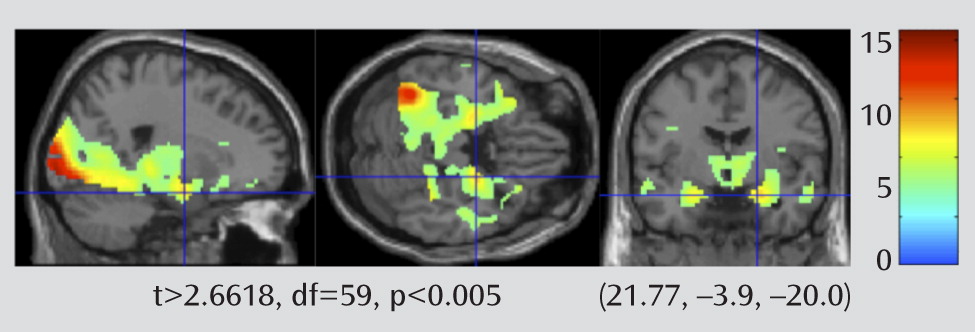
To verify that the fMRI task engaged the expected limbic regions in this sample of elderly participants, we first looked at the overall effect of task on the entire group (late-life depression patients and elderly comparison subjects) (
Figure 3). We set the map threshold at t>2.6618, df=59, p<0.005, uncorrected. The map points out areas where there is greater BOLD signal associated with performing the faces blocks relative to the shapes blocks. This map shows a pattern similar to that observed in other studies using this task with healthy comparison subjects that include the elderly (
18).
The amygdala bilaterally, the insula, and the rostral cingulate approached significance with family-wise error rate with a small-volume correction.
Table 2 and
Table 3 show the family-wise error corrections for the a priori regions of interest for the main effect of task contrast as well as for the comparisons with depression and WMH burden. These tables include values that meet a threshold of p<0.01, corrected.
The Main Effect of Depression
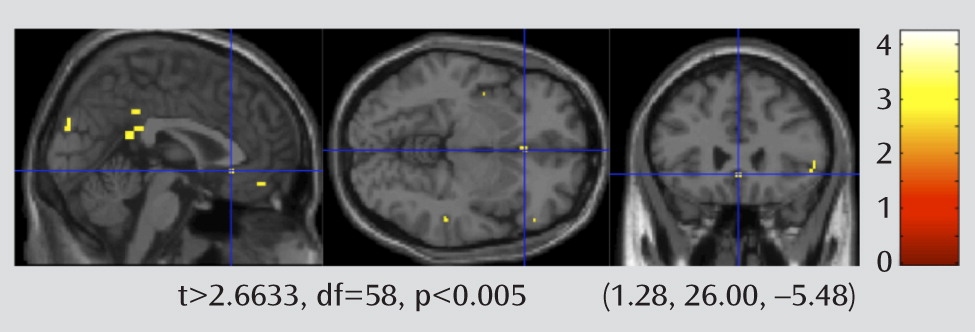
The fMRI contrast maps for faces > shapes for each participant were used to compare the difference in BOLD response between the depressed and nondepressed groups. The results of this random-effects between-group analysis is shown in
Figure 4. We set the threshold for the two-sample t test map at a voxel-wise t>2.6633, df=58, p<0.005. A family-wise error rate small-volume correction was then applied for the a priori regions of interest (rostral cingulate, insula, and amygdala).
The imaging shows relatively greater activity during the faces task in the rostral anterior cingulate in depressed patients (
Figure 4). The activity in the rostral cingulate is primarily in the subgenual component (Brodmann's area 25), an area associated with midlife depression that has served as the target for deep brain stimulation (
13). The activity in the rostral cingulate remained significant after correcting for multiple comparisons using family-wise error with small-volume correction.
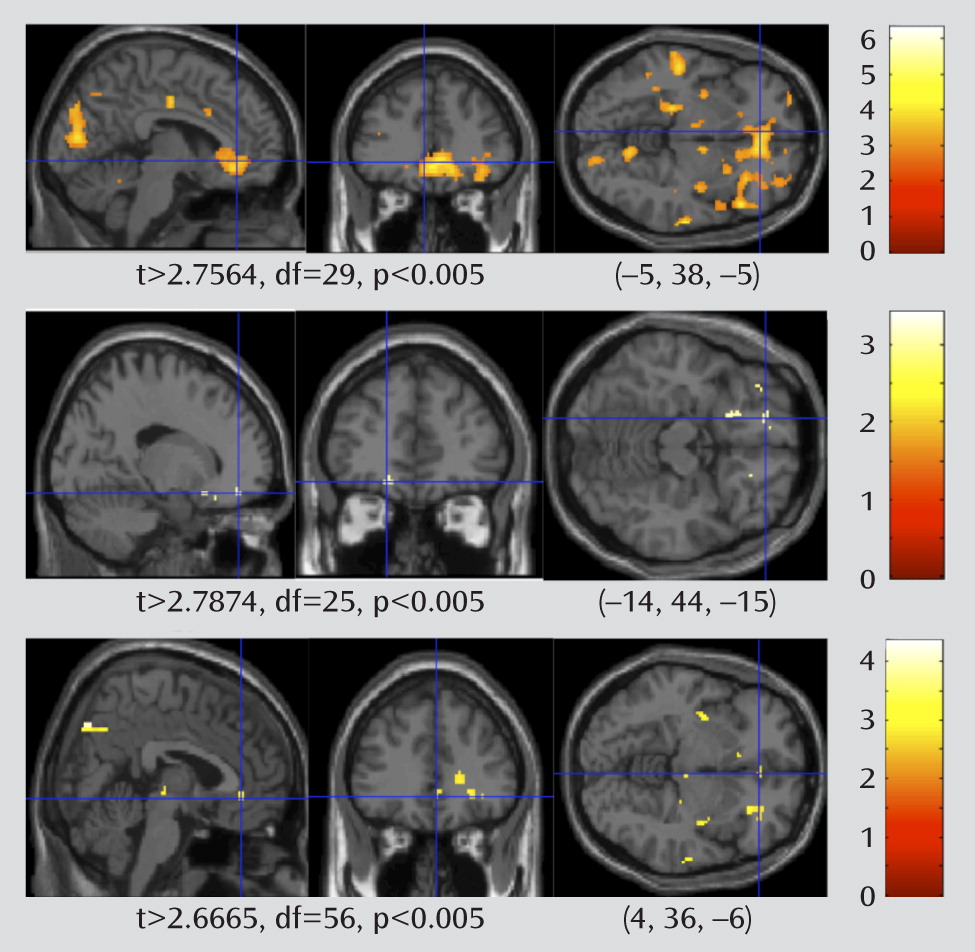
The Effect of WMH Burden in Participants With and Without Late-Life Depression
To test whether higher WMH burden is associated with greater limbic BOLD signal, we split the group of depressed patients into those with high and low WMH burden, and we contrasted the fMRI activation in these two groups. We also performed the same contrast in the nondepressed elderly comparison subjects.
In these analyses, the fMRI faces > shapes contrast maps were compared using a random-effects between-group analysis, comparing those with a low WMH burden to those with a high WMH burden. The results of the two-sample t test for the depressed and comparison groups are shown in
Figure 5. There is a more robust effect of WMH burden in the depressed group relative to the nondepressed group. In the depressed group, the rostral cingulate shows greater BOLD response in those late-life depression patients with high WMH. There are no areas that show significantly greater activity in the low-WMH-burden patients (at t>2.744, df= 31, p<0.005, uncorrected). In addition to the rostral cingulate regions of interest, the insula also shows greater BOLD response in the high-WMH-burden participants. The amygdala showed higher BOLD response with greater WMHs, but the association did not reach statistical significance. Results of fMRI based on the regions of interest are summarized in
Table 2. These results suggest that the association of WMH burden with BOLD activity appears to be specific for elderly patients with depression. This supports the view that the burden of WMHs is associated with the functional changes in elderly patients with clinical depression, despite the fact that white matter damage may also occur in nondepressed elderly people.
Discussion
We examined the association of WMH burden with the pattern of functional BOLD response during affective processing in late-life depression. The primary aim of this study was to test whether the often-reported white matter changes in late-life depression are associated with functional brain changes during affective processing. Our results show that the late-life depression patients showed a pattern of greater limbic fMRI activity relative to nondepressed elderly comparison subjects. This pattern was accentuated in the late-life depression patients with the highest WMH burden. Moreover, a significant interaction between depression and WMH burden was observed with respect to their association with fMRI activity, indicating that the association of WMHs with greater limbic fMRI activity is particularly pronounced in the depressed group. This cross-sectional study shows association, rather than causation, but it nonetheless supports the vascular depression hypothesis that ischemic white matter changes contribute to the pathogenesis of late-life depression. One possible interpretation is that the white matter changes in late-life depression disrupt the ability of the prefrontal cortical structures to modulate the hyperactive limbic structures through altered connectivity.
In this study, the difference in WMH burden in the late-life depression patients relative to comparison subjects fell short of significance. This was somewhat unexpected, as higher WMH burden in late-life depression has been reported in many (but not all [
5]) previous reports. We believe the observation of WMH differences between the depression and comparison groups depends on factors such as how closely subjects are matched on cerebrovascular risk, heterogeneity of the depressed sample, and power to detect differences in groups closely matched on cerebrovascular risk. If the groups are closely matched on cerebrovascular risk, then we could expect similar levels of WMH burden between the groups. However, matching on cerebrovascular risk can bias subject selection. Since depression itself contributes to several of the most important cerebrovascular risk factors (e.g., smoking, hypertension, and diabetes), matching on this risk means oversampling cerebrovascular risk in the comparison subjects. This limits the ability to generalize to the population, although it allows for the identification of fMRI signals that are differentially associated with depression and WMH burden. Even though the WMH burden in our group of late-life depression patients does not differ considerably from our group of nondepressed elderly comparison subjects, there was enough variability in WMH burden to examine the relationship of WMH burden with fMRI patterns associated with depression.
The role of structural brain changes in late-life depression has been the focus of much research on the neurobiology of depression in the elderly and has served as the focus of the vascular depression hypothesis (
1,
30). These structural imaging markers have been useful as potential biomarkers for predicting treatment; several studies have reported a correlation between WMH burden and treatment response in late-life depression (
4). The association of WMH with depression is of particular importance in late life, as age is strongly associated with WMH burden (
31). In midlife depression, the functional neuroimaging markers, primarily pretreatment rostral cingulate hyperactivity, have been predictive of a robust response to antidepressants. This observation, however, has not been reported in late-life depression (
14). In fact, the opposite pattern has been observed, with lower rostral cingulate metabolism being associated with better treatment response in late-life depression.
Our observation that late-life depression rostral cingulate hyperactivation is associated with greater WMH burden suggests that late-life depression may have different functional predictors of treatment than those observed in midlife depression. These results are primarily from FDG PET studies, indicating higher metabolic activity in these regions. Our present study examined BOLD fMRI activity, an indirect marker of primarily task-related neural activation (
32). Since age-related structural brain changes (in this case, white matter lesion load) are associated with worse treatment response (
4), we could predict that rostral cingulate hyperactivation in the elderly (which is associated with WMH burden) would be associated with poor treatment response. Studies are needed to clarify these relationships, which may point to a neurobiological feature that differentiates late-life depression from midlife depression.
The localization of the fMRI results in WMH contrast parallels the findings in the depression > comparison group contrast. In both of these results, the fMRI contrast approaches greater statistical significance in the rostral cingulate than in the amygdala. Thus, the similarity of the localization in these two contrasts points out the possible functional association of WMHs with late-life depression. The depression > comparison group contrast has a relatively small effect, which suggests that the limbic activity in late-life depression seems smaller than the more robust findings reported in younger patients. However, other differences in the fMRI signal associated with age (e.g., changes in the hemodynamic response function) may also account for the difference in this fMRI contrast.
There are several aspects of this study that limit the interpretation of our results. We focused only on elderly participants and used a cross-sectional design. Although we feel these results are consistent with a model in which white matter damage in the elderly disrupts top-down control of limbic activity, we have only demonstrated cross-sectional associations and cannot make causal inferences about the correlation between white matter damage and limbic hyperactivation. Directionality may be reversed, with depression being a risk factor for cerebrovascular changes and worsening of white matter disease (
33). Longitudinal imaging studies of depression across the lifespan are necessary for distinguishing among these possibilities.