Cognitive impairment is considered to be a core deficit of schizophrenia (
1). Subjects with schizophrenia show worse functioning than matched healthy subjects in several cognitive domains, e.g., memory (
2) and executive functioning (
3). There is an urgent need for systematic research in this area because such deficits 1) profoundly affect the long-term outcome (
4), 2) do not remit between episodes, placing an enormous burden on the families (
5), 3) have little correlation with positive symptoms (
6), and 4) are minimally affected by current medications (
7).
Genetic and environmental factors may contribute to cognitive impairments. Among the environmental factors, emerging evidence supports the role of neurotropic viruses, e.g., herpes viruses. These agents can cause lifelong infection in the brain, with latency and reactivation cycles associated with neuronal damage or dysfunction (
8,
9). Elevated prevalence of seropositivity for herpes simplex virus subtype 1 (HSV1) in schizophrenia is inconsistent (
10,
11), and whether such agents confer risk for schizophrenia is uncertain. Nonetheless, there are replicable associations of HSV1 exposure with cognitive impairments (
12,
13) and low prefrontal cortex volume (
14,
15). In addition, low cross-sectional prefrontal gray matter has been correlated with impaired performance on the trail-making tests among HSV1-exposed schizophrenia subjects (
15). These associations have medium to large effect sizes and appear to be more prominent in schizophrenia than in comparison subjects. Separately, we have noted a different pattern of association of cognitive impairments with exposure to cytomegalovirus, another virus within the herpes family (
13). However, these associations do not necessarily prove etiological links. A natural history of lifelong periodic reactivations leading to longitudinal decline in cognitive functioning and related neurobiological measures could further support the role of HSV1 exposure in cognitive impairments.
In this study, our goals were to test the hypothesis that HSV1 exposure would be associated with longitudinal changes in executive functioning and longitudinal gray matter loss in regions implicated in the regulation of executive functioning. We examined executive functioning and gray matter changes over 1 year among subjects with first-episode schizophrenia who were antipsychotic naive at baseline, and we compared their changes to those in healthy subjects. We included cytomegalovirus exposure to investigate the relative specificity of the associations with HSV1 exposure.
Results
Demographic and Clinical Characteristics
At baseline, the study group comprised 26 subjects with schizophrenia and 38 healthy subjects. Nearly two-thirds of the subjects with schizophrenia (65%) were male, and their mean age was 23.59 years (SD=7.82). Their mean illness duration—the time between the onset of the first psychotic symptom and the time of evaluation—was 2.12 years (SD=2.26). In the healthy comparison group, 55% of the subjects were male, and the mean age was 23.05 years (SD=4.85). The majority of subjects were available for follow-up (
Table 1). The subjects with schizophrenia differed from the healthy subjects with regard to score on the Hollingshead index of socioeconomic status (schizophrenia: mean=36.06, SD=12.93; healthy: mean=44.80, SD=10.44; t=2.98, df=62, p=0.004) but not age, sex, or serostatus at baseline, 6 months, or 1 year. There were no significant differences between the HSV1-seropositive and -seronegative schizophrenia subjects in scores on the Scale for the Assessment of Positive Symptoms (SAPS) (seropositive: mean=28.62, SD=10.77; seronegative: mean=24.00, SD=11.05) and the Scale for the Assessment of Negative Symptoms (SANS) (seropositive: mean=40.85, SD=8.07; seronegative: mean=46.85, SD=40.85) at baseline (all p>0.11) or at follow-up (positive symptoms: 7.60 [SD=6.79] versus 9.80 [SD=7.12]; negative symptoms: 39.00 [SD=11.40] versus 33.60 [SD=9.42]) (p>0.37 in all cases). Age, sex, illness duration, and Brief Psychiatric Rating Scale (BPRS), SANS, and SAPS scores did not differ between individuals who were followed up for 1 year and those who were not followed up (p>0.31 in all cases). However, the individuals not available at 1 year did have lower socioeconomic status (t=2.25, df=62, p=0.03).
MRI studies were completed by 18 schizophrenia subjects and 24 comparison subjects. There were no significant differences between these groups in age (schizophrenia: mean=24.00 years, SD=6.82; comparison: mean=23.75, SD=6.09), sex, or socioeconomic status. Age, socioeconomic status, sex, illness duration, and scores on the BPRS, SANS, and SAPS did not differ between participants included and excluded from the imaging analyses (p>0.40 in all cases).
Structural Imaging
Cross-sectional morphometric studies did not show that the interaction between diagnosis and HSV1 status had an effect on gray matter volume across the entire study group, at either baseline or follow-up. When the schizophrenia and healthy subjects were analyzed separately, the HSV1-seropositive schizophrenia subjects had a smaller gray matter volume in combined Brodmann's areas 8, 9, and 32 than did their seronegative counterparts, at baseline (seronegative: mean=4.92 cc, SD=0.20; seropositive: mean=4.50, SD=0.14; t=3.23, df=16, p=0.005) and at follow-up (seronegative: mean=4.67 cc, SD=0.28; seropositive: mean=4.26, SD=0.24; t=5.18, df=16, p=0.00009). This was not the case for the healthy subjects (p>0.16 at both times). The HSV1-related differences in gray matter volume did not differ significantly between the schizophrenia and comparison groups.
Deformation fields analysis of the longitudinal morphometric studies revealed that schizophrenia subjects as a group showed a reduction in the bilateral posterior cingulate gyri (left: T=11.43, false discovery rate p=0.002; right: T=8.49, p=0.005) over 1 year. The estimated mean gray matter reduction in the posterior cingulate gyrus at 1 year was 0.82 cc on the left and 0.18 cc on the right. Prefrontal gray matter volumes did not show longitudinal changes, and healthy subjects did not show significant gray matter changes in any region during this period.
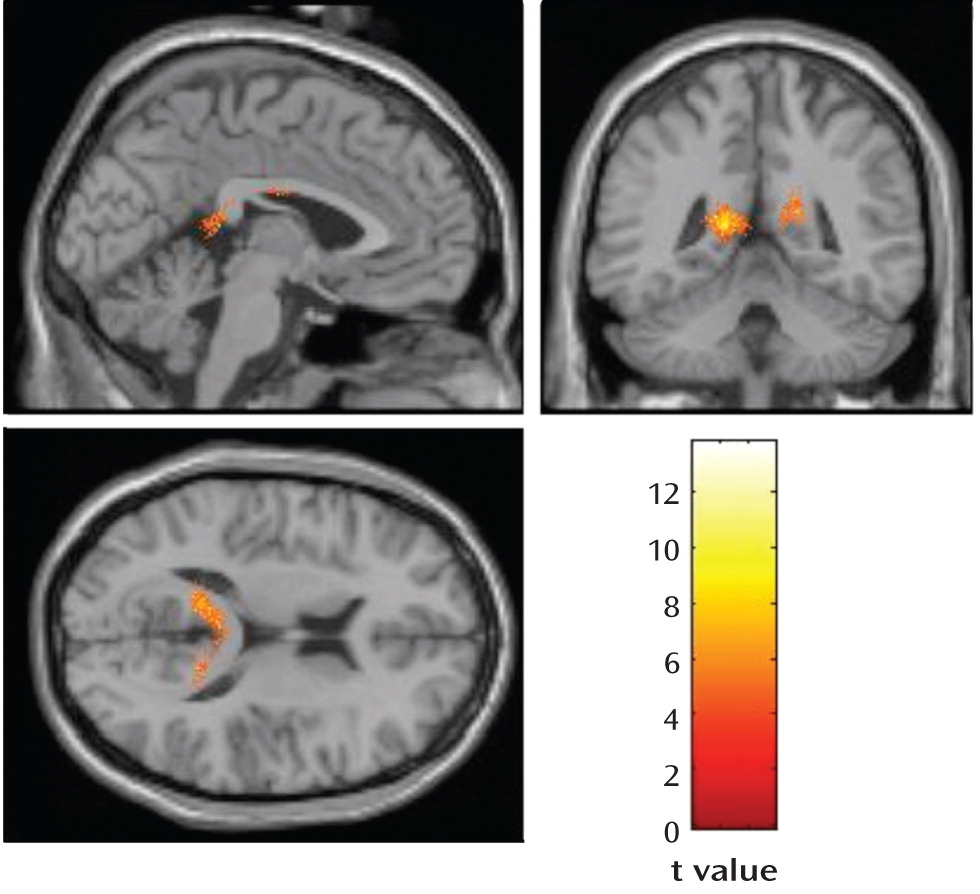
Within the HSV1-seropositive schizophrenia subjects, the deformation fields analysis revealed a significant reduction over 1 year in gray matter volume in the left posterior cingulate gyrus and a nearly significant change for the right posterior cingulate gyrus (
Figure 1). The mean estimated reduction in gray matter volume was 1.60 cc in the left posterior cingulate gyrus and 0.66 cc on the right. Significant gray matter loss during the same period was not observed in the seronegative schizophrenia subjects or in either the HSV1-seronegative or -seropositive healthy subjects.
Executive Functioning
Categories completed.
Using the mixed-effects model, we evaluated main effects on the number of categories completed on the Wisconsin Card Sorting Test. The effects of diagnosis, follow-up duration, HSV1 status, and cytomegalovirus status were examined. Next, diagnosis-by-HSV1, diagnosis-by-time, and three-way diagnosis-by-serostatus-by-time interactions were examined separately for HSV1 and cytomegalovirus (
Table 2).
In the entire study group, the main effects of diagnosis and time suggested that the subjects with schizophrenia completed fewer categories than the healthy subjects and that the group as a whole completed more categories at the 1-year follow-up than at baseline. A nearly significant effect of HSV1 serostatus (p=0.07) suggested that the HSV1-exposed subjects (schizophrenia and comparison subjects combined) completed fewer categories than did the unexposed subjects. Significant interactions between time and HSV1 status and between time and cytomegalovirus status in the combined group indicated that fewer categories were completed by subjects exposed to either HSV1 or cytomegalovirus than by those unexposed.
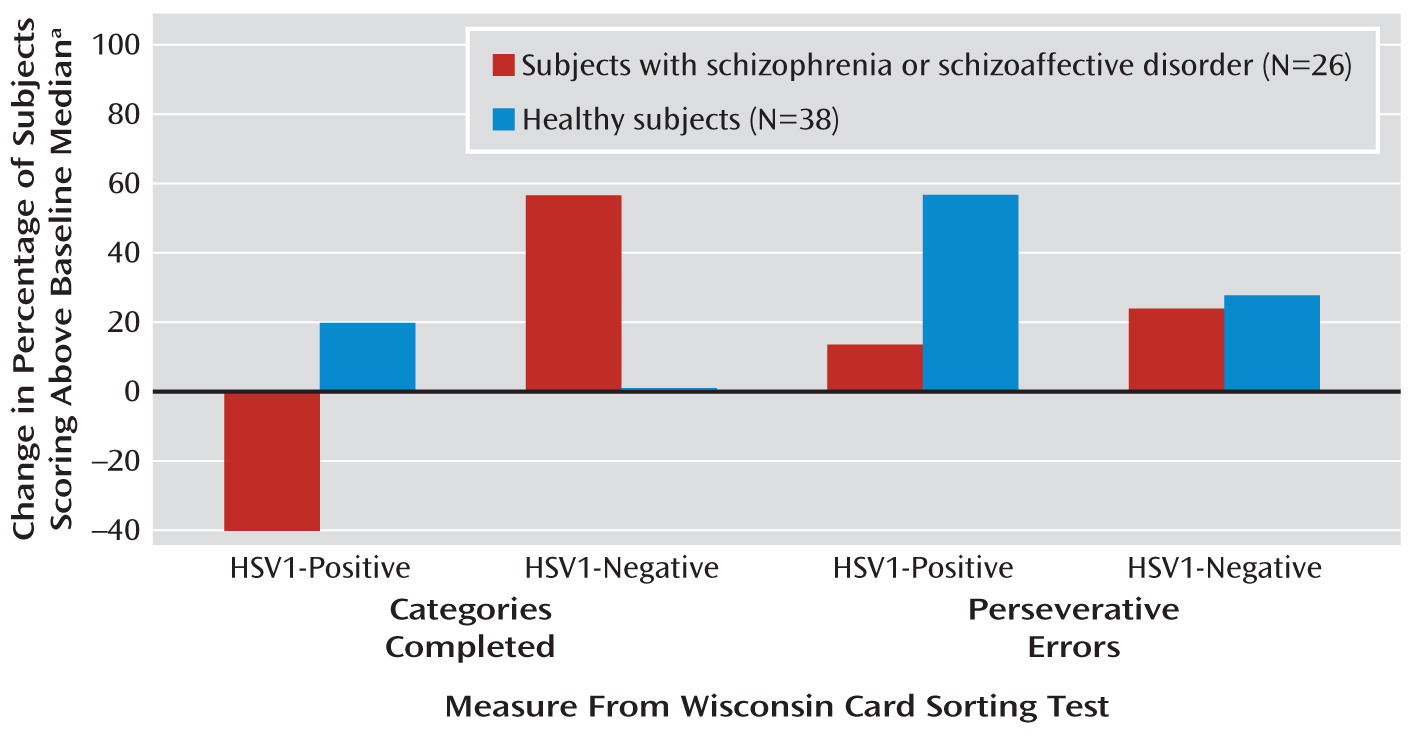
Within the diagnostic groups, the number of HSV1-negative schizophrenia subjects who could complete more than the median number of categories at baseline for the study group (i.e., more than five categories) increased by 57% over 1 year (
Figure 2). In contrast, the number of seropositive schizophrenia subjects who completed more than five categories fell by 40% over 1 year. The healthy comparison subjects did not show such changes, possibly because of their near-ceiling performance at baseline (
Figure 2). Cytomegalovirus-seropositive subjects showed a similar pattern during the follow-up (data not shown). The three-way interaction of time, diagnosis, and cytomegalovirus status was significant (
Table 2). After the cytomegalovirus association was included in the model, the three-way interaction of time, diagnosis, and HSV1 status remained highly significant (
Table 2). These models accounted for baseline differences as well as variance contributed by cytomegalovirus status.
Perseverative errors.
A separate mixed model examined the main effects and interactions for perseverative errors in the same manner as for the categories completed (
Table 2). In the combined study group (schizophrenia plus healthy subjects), we observed main effects of diagnosis and time. The subjects with schizophrenia committed more errors than the healthy subjects, and over time the group as a whole committed fewer errors. We did not observe a main effect of cytomegalovirus or HSV1 status. There were nearly significant interactions between HSV1 status and diagnosis and between HSV1 status and time but not between diagnosis and cytomegalovirus status. Individuals in the combined study group who were exposed to HSV1 did not commit more errors than those who were unexposed.
Over 1 year, the number of HSV1-seronegative schizophrenia subjects who committed fewer than the median number of errors for the study group (fewer than nine) increased by 25%, compared with 14% in the HSV1-seropositive schizophrenia subgroup (
Figure 2). Cytomegalovirus-seropositive subjects did not show such associations. The final model that accounted for baseline differences and cytomegalovirus exposure showed a significant three-way interaction of time, diagnosis, and HSV1 status, indicating a greater reduction in perseverative errors among the HSV1-seronegative schizophrenia subjects than among those who were seropositive.
Correlations of Changes in Executive Functioning and Gray Matter Volumes
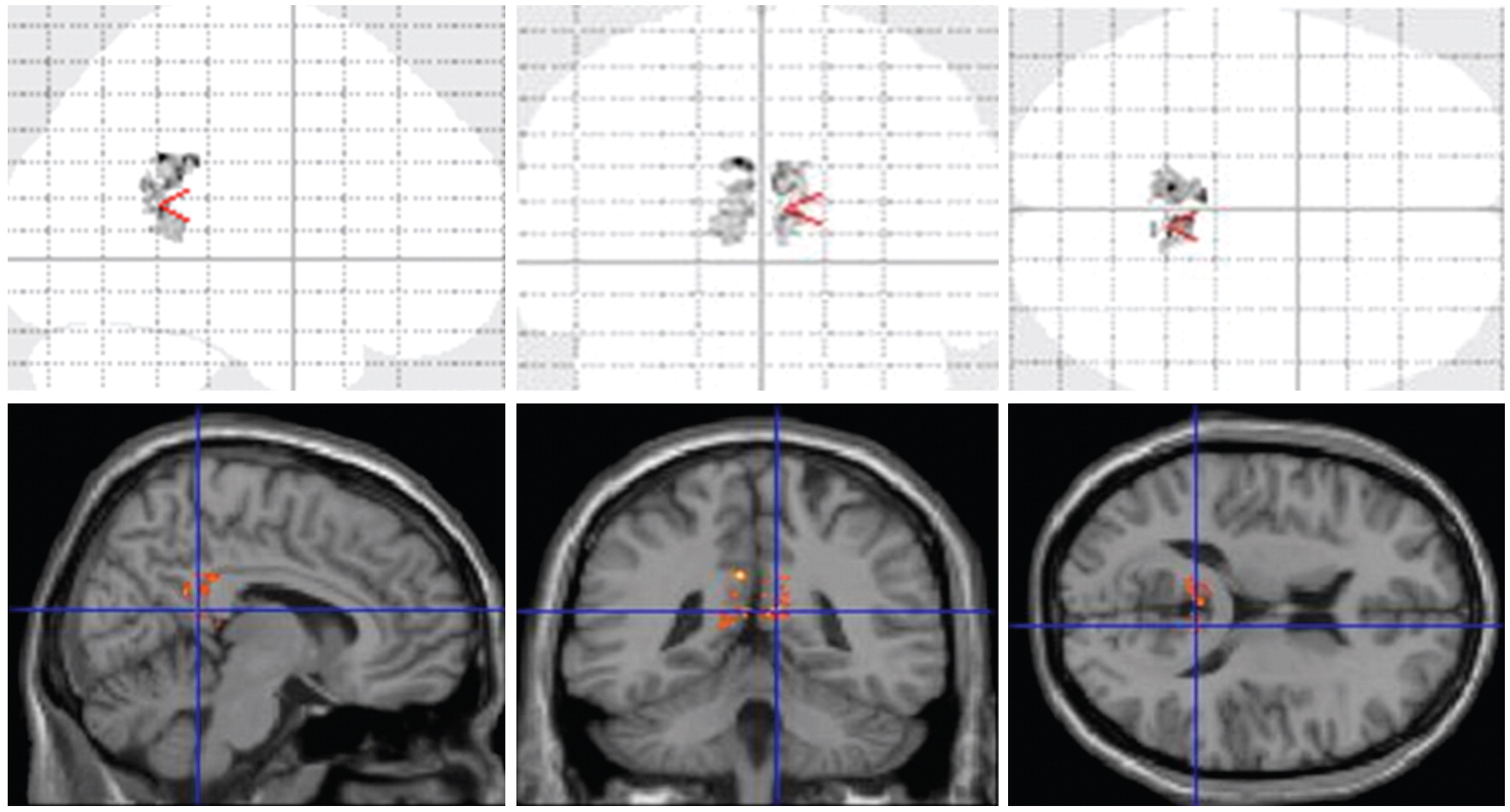
Post hoc multiple regressions on the combined study group (schizophrenia and healthy subjects) showed a significant correlation between change in the number of perseverative errors over 1 year and the change in posterior cingulate gyrus volume (Pearson r=0.60, N=42, p=0.0002). This revealed that increases in perseverative errors correlated with volume loss in the posterior cingulate gyrus. These observations were most prominent among the HSV1-seropositive schizophrenia subjects (
Figure 3). We did not observe significant correlations with the change in the number of categories completed.
Discussion
We observed significant loss of posterior cingulate gyrus gray matter over 1 year among HSV1-seropositive subjects with schizophrenia. Consistent with the volume loss was the finding that more HSV1-seropositive than -seronegative schizophrenia subjects showed a decline in performance on executive functions over 1 year. Specifically, more HSV1-seropositive schizophrenia subjects had a decline in Wisconsin Card Sorting Test categories completed and an increase in perseverative errors compared with HSV1-seronegative schizophrenia subjects. Although cytomegalovirus exposure was associated with significant reduction in the number of categories completed (not perseverative errors), it did not account for the HSV1-associated changes. The neuropsychological and structural MRI findings appear to be convergent. The posterior cingulate gyrus is thought to be involved in the regulation of executive control and working memory (
24). However, the structure-function relationship can be complex (
25). Previous follow-up studies of survivors of HSV1 encephalitis indicate continued gray matter loss and cognitive impairments (
26). To our knowledge, this pilot study is the first one to show that HSV1 exposure is associated with longitudinal gray matter loss and a decline in executive functioning without a history of acute encephalitis. Individuals with schizophrenia may be particularly susceptible to such progression.
There are several plausible explanations for the observed changes. In the rodent and rabbit models of CNS HSV1 exposure, latent infection and reactivation directly affected functioning through neuronal death or dysfunction. Neuronal death resulted from apoptosis (
27). Neuronal dysfunction during reactivation and latency resulted from modulation of apoptosis (
8) and autophagy (
9), host cell translational shutoff (
28), oxidative damage (
29), and/or neurotransmitter alterations (
30). Even with peripheral infections, HSV1 could alter neurotransmission through release of cytokines, especially chemokines (
31), which may be elevated in HSV1-exposed individuals (
31). Human studies support some of these observations (
32). These processes occur throughout the life of an infected person.
The precise reasons for loss of posterior cingulate gyrus gray matter are unknown. The posterior cingulate volume loss in longitudinal studies of schizophrenia is inconsistent (
33,
34), possibly because sensitive computational approaches were not used. Using deformation fields analysis, we noted longitudinal gray matter loss in schizophrenia regardless of serostatus. In our previous cross-sectional study, we did not observe posterior cingulate gyrus volume differences at baseline for schizophrenia and healthy subjects across HSV1 serostatus (
14). Another study indicated lower than normal baseline posterior cingulate gyrus volume among HSV1-positive schizophrenia subjects that correlated with trails test performance (
15). In this study, both deformation fields analysis and extracted volumes showed smaller gray matter volumes in the HSV1-seropositive subjects with schizophrenia, but volume differences observed on extracted volumes should be viewed with caution because they are based on rule-based boundaries assigned by the Talairach database and may not precisely represent the absolute anatomy of the regions (
17). Longitudinal changes have been noted in animals and in patients with successfully treated HSV1 encephalitis. In animals, long-term HSV1 exposure followed by acyclovir treatment, but not short-term exposure, was associated with cingulate cortex neuronal loss (
35). Similarly, recovering patients with acyclovir-treated HSV1 encephalitis continue to show posterior cingulate gyrus gray matter loss (
26). HSV1 infection may also activate proinflammatory cytokines (
36), which are associated with decreased posterior cingulate gyrus volume (
37). We did not observe longitudinal prefrontal cortex changes, possibly because our relatively small group of structural scans might have shown significant changes for the region with the largest effect size. For regions with smaller effects (Cohen's d of 0.2 or lower), we need to double the number of subjects.
The sensitive and specific nature of the serum antibody assays used here reliably indicates prior exposure to the neurotropic agents, providing a noninvasive and sensitive proxy for brain infection. The presence of HSV1 DNA in the trigeminal ganglia in human postmortem studies significantly correlates with peripheral evidence of HSV1 infection (
38). However, there is no reliable method for directly demonstrating current brain infection; polymerase chain reaction assays detect viral DNA in the CSF for only a proportion of patients with acute encephalitis (
39). Further, serological assays cannot precisely indicate the timing or duration of the infection, e.g., prenatal versus postnatal exposure. There are no systematic human studies on exposure at different developmental periods and specific differential effects on brain structure or function and cognitive performance. Animal studies suggest that prenatal exposure (particularly within the first few weeks) may be more important than postnatal exposure for schizophrenia risk (
40). However, it is unclear whether the same holds for cognitive impairments. It is possible that the HSV1-positive individuals in this study were infected in the prenatal or perinatal period, with the current assays indicating past exposure and recrudescences. Thus, seropositive individuals may reflect the impact of acute and/or chronic exposure, as well as alterations in a number of neurodevelopmental processes. Since several cellular and molecular changes have been demonstrated following HSV1 exposure, a person exposed for a longer time can be expected to show more abnormalities.
It is unclear why the HSV1-positive schizophrenia subjects but not the HSV1-positive healthy subjects showed significant longitudinal changes. In contrast to the individuals with schizophrenia, the healthy subjects performed optimally at baseline and thus may have had greater compensatory reserve. The schizophrenia group may have had more prolonged exposure than the comparison subjects. Alternatively, variations in the host immune response or exposure to other infectious agents could explain between-group differences.
On the basis of existing evidence from cross-sectional studies that showed impaired working memory and executive functioning, we hypothesized longitudinal changes in executive functioning. Our analyses support this prediction, but there are several limitations. Practice effects might explain part of the variance. Since all subjects took the tests at both time points, it is unlikely that the practice effects solely explain these observations. Specifically, practice effects may explain improved performance for the HSV1-negative schizophrenia subjects (
Figure 2), but they cannot explain the decline in functioning observed in the HSV1-exposed subjects. Differences in medications between the exposed and unexposed patients may explain some of the results, though the severity of positive and negative symptoms in the HSV1-seropositive group did not change between baseline and follow-up. All of the subjects evaluated at baseline were not available for follow-up evaluations, but the proportions of seropositive schizophrenia and healthy subjects did not change significantly. Further, clinical and demographic characteristics did not differ between those who were followed up and those who dropped out, except for socioeconomic status, and all results were controlled for this difference. Thus, differential attrition may not significantly account for the observed differences.
In conclusion, we report longitudinal changes in posterior cingulate gyrus gray matter volume and executive functioning among schizophrenia subjects exposed to HSV1, after variance due to cytomegalovirus exposure and other potential confounds was accounted for. Significant changes were not observed for the imaging or cognitive measures among HSV1-seronegative schizophrenia subjects or among the healthy comparison subjects. These results are important because of consistent and substantial correlations between cognitive impairments and long-term outcome in schizophrenia. Cohort-based studies can clarify whether these associations reflect an etiological effect of HSV1 exposure. Such studies are justifiable, as effective medications to treat HSV1 infection are available.