Cannabis is the most commonly used illicit substance among adolescents, and rates of use are increasing. One quarter of high school seniors are current cannabis users, and 7% use daily (
1). Adolescents are particularly prone to adverse consequences of cannabis use and progression to dependence (
2–
5), but existing cessation treatments are associated with low abstinence rates (
6–
8). A potential strategy to enhance outcomes is to use pharmacotherapy to complement psychosocial treatment, but little research has been conducted on this approach in adolescents. Even in adults, investigation of pharmacotherapy for cannabis dependence has been limited, and no effective medications have been identified (
9).
The antioxidant
N-acetylcysteine (NAC), an
N-acetyl prodrug of the naturally occurring amino acid cysteine, is widely available as an over-the-counter supplement. Research interest in NAC has grown amid increasing evidence of the role of the neurotransmitter glutamate in addiction (
10–
12). Animal models have demonstrated that chronic drug self-administration down-regulates the cystine-glutamate exchanger in the nucleus accumbens and that administration of NAC up-regulates this exchanger, normalizing a drug-induced pathology and reducing reinstatement of drug seeking (
10,
13). Preclinical and preliminary clinical studies further support a potential treatment role for NAC (
14–
21). Other clinical studies suggest that NAC, via glutamate modulation and a number of other proposed mechanisms, may be efficacious across a variety of psychiatric conditions (
22).
Given these findings, and in light of the need for improved adolescent cannabis cessation treatments, we examined NAC as a candidate pharmacotherapy. After completing an encouraging open-label pilot trial (
23), we conducted a double-blind randomized placebo-controlled trial of NAC in cannabis-dependent adolescents. To critically judge NAC as a complementary treatment, we evaluated it in the context of contingency management, an efficacious youth-targeted cannabis cessation psychosocial treatment (
24,
25). We hypothesized that treatment with NAC, relative to placebo, when added to contingency management and brief weekly cessation counseling, would be associated with higher rates of abstinence, as measured by the odds of negative urine cannabinoid test results during treatment.
Method
Trial Design
Treatment-seeking cannabis-dependent adolescents were randomly assigned, in a 1:1 parallel group allocation, to receive a double-blind 8-week course of NAC (1200 mg) or placebo twice daily, along with contingency management and brief weekly cessation counseling. A follow-up assessment was conducted 4 weeks after end of treatment. Urine cannabinoid testing (U.S. Screening Source, Inc., Louisville) was conducted at all visits. The Investigational New Drug application for this study was approved by the Food and Drug Administration (FDA). The study procedures were approved by the university institutional review board and were in accord with the Helsinki Declaration of 1975.
Participants
To enroll in the study, adolescents had to be 13 to 21 years old, use cannabis regularly (≥3 days/week on average), meet criteria for cannabis dependence, express interest in cannabis cessation treatment, not be enrolled in substance use treatment, have no current comorbid substance dependence aside from nicotine, have no acutely unstable psychiatric or medical illness, have no history of adverse reaction to NAC, and not be taking carbamazepine or nitroglycerin; in addition, female participants could not be pregnant, and if sexually active, had to be using birth control.
Recruitment occurred primarily through clinical referrals and local media (e.g., flyers, newspaper announcements, online advertisements). If an initial telephone screen suggested potential eligibility, adolescents were scheduled for an informed consent and baseline assessment visit. After receiving a complete description of the study, all participants age 18 years or older provided written consent; written parental consent and participant assent were obtained for those under age 18.
General Procedures
All procedures were conducted at the university research clinic. At the baseline visit, comprehensive psychiatric and substance use diagnostic assessment (
26–
28), physical examination, and laboratory testing (urine pregnancy and drug tests) were performed. Timeline follow-back methods were used to assess self-reported cannabis and other substance use (
29).
Eligible participants were given adolescent-targeted cannabis information brochures (
30), were enrolled in a contingency management intervention (see below), and were randomly assigned to a treatment group. Participants were seen in clinic weekly during the 8-week medication trial and returned for posttreatment follow-up 4 weeks after end of treatment. At all visits, the study physician or physician assistant provided brief individual cessation counseling (<10 minutes) and adverse event assessment, and participants submitted urine samples for cannabinoid testing.
Interventions
Medication.
Enrolled participants were randomly assigned to double-blind treatment (NAC, 1200 mg, or placebo twice daily), stratified by age (<18 or ≥18) and baseline cannabis use (using <20 or ≥20 of the past 30 days). The university investigational drug service oversaw randomization, encased medications in identical-appearing capsules, and dispensed them in weekly blister packs with specific instructions on when to take each dose. Participants, investigators, and clinical staff remained blind to treatment assignment throughout the study. To enhance the blind, a small amount of NAC powder was applied to the inside of all blister packs so that both NAC and placebo packs would contain the scent of NAC. No formal assessment of the integrity of the blind was conducted.
Contingency management.
A twice-weekly contingency management intervention, separately targeting participant retention and cannabis abstinence and modeled on established methods (
24), was implemented during the medication trial. An escalating reinforcement schedule, in which participants were able to earn increasing contingent rewards over successive displays of desired behavior (adherence with appointments and procedures; cannabis abstinence as measured by instant urine cannabinoid testing), was used. One weekly evaluation occurred during the week's scheduled clinic visit, and the other was a “drop-in” on a separate day of the week. For adherence and abstinence, the initial contingent reward was $5 (cash) for each. For each successive visit at which the participant was adherent or abstinent, the reward increased by $2 ($7, then $9, and so on). If a participant subsequently failed to adhere to study procedures or tested positive for cannabis use, he or she did not receive any reward at that visit, and the contingent reward value for the next session was reset to the baseline of $5. If, at a given visit, a participant tested positive but adhered to study procedures, he or she collected the adherence reward as scheduled but was not eligible for the abstinence reward.
Cessation counseling.
The physician or physician assistant, in the context of medication management, provided nonmanualized brief (<10 minutes) cessation counseling at all clinic visits, incorporating educational, motivational, and cognitive-behavioral elements.
Outcome Measures
Efficacy.
Urine cannabinoid testing at baseline, during weekly clinic visits, and at the posttreatment follow-up, was conducted as the primary biological measure of cannabis use. Self-reported cannabis use was collected by timeline follow-back methods.
Safety and tolerability.
A thorough safety evaluation was conducted at each clinic visit, including a physician or physician assistant evaluation of adverse events using an open-ended interview and a comprehensive structured review of systems (
31); urine pregnancy testing for female participants; and measurement of vital signs.
Adherence.
Medication diaries and weekly pill counts (inspection of blister packs and documentation of missed doses) were used to measure adherence.
Statistical Analysis
The primary hypothesis was that participants in the NAC group would have higher odds than those in the placebo group of having negative weekly urine cannabinoid test results during treatment. An intent-to-treat approach including all randomized participants was used. In all analyses, participants who were lost to follow-up or were absent from visits were coded as having a positive urine cannabinoid test at every missed visit.
The study was powered to detect a 50% rate of negative urine cannabinoid tests in participants receiving NAC, compared with 25% in those receiving placebo. These estimates were derived from a previous trial of pharmacotherapy to complement contingency management targeting cocaine dependence (
32). Setting the type I error rate to 0.05, a sample of 58 participants per treatment group was deemed necessary to yield 80% power. No interim efficacy analyses were conducted.
Standard descriptive statistics were used to summarize the general demographic and clinical data. Group differences in continuous characteristics were assessed using t tests, and differences in categorical characteristics were assessed using normal (Pearson's) chi-square tests.
The efficacy of NAC compared with placebo, along with contingency management and weekly brief cessation counseling, in fostering abstinence from cannabis was analyzed over the 8-week treatment and at the follow-up 4 weeks after end of treatment. A repeated-measures logistic regression model using the methods of generalized estimating equations (
33) was applied to assess the overall treatment effect on urine cannabinoid test results during active treatment. Working correlation structures were independently compared, and the final model structure was chosen using the quasi-likelihood under the independence model criterion (
34). Odds ratios and asymptotic 95% confidence intervals (CIs) were computed. Additionally, a preplanned logistic regression model was used to analyze the odds of a negative cannabinoid test at the posttreatment follow-up.
Exploratory analyses of selected secondary efficacy measures were conducted. Time to first negative urine test was examined using a Cox proportional hazards model with the baseline visit set as the time baseline. The assumption of proportional hazards was assessed by including an interaction between the treatment group assignment variable and the log-transformed time-to-event variable. No violations of the assumption were determined. End-of-treatment abstinence was assessed via a logistic regression model using the intent-to-treat sample (N=116) in which participants with missing data were assumed to be nonabstinent. An analysis of covariance model was used to test for differences in the proportion of days of cannabis use throughout treatment.
All study models were adjusted for baseline urine cannabinoid test results and assessed for possible confounding and effect modification of age, weight, gender, race, years of cannabis use, number of previous quit attempts, and presence of psychiatric comorbidities. Baseline demographic and clinical characteristics were independently tested for association with efficacy outcome, and those significantly associated were included as predictors in adjusted models. Results are presented as odds ratios with 95% CIs.
Adverse event rates were compared between treatment groups using Pearson's chi-square tests. Study completion was compared using logistic regression. Cox proportional hazards regression was used to assess the effect of demographic and clinical characteristics and treatment assignment on time to study dropout. The assumption of proportional hazards was assessed similarly to the aforementioned efficacy model. No violations of the assumption were determined.
No adjustments for multiple testing were made, as they are known to reduce statistical power and increase the probability of accepting a null hypothesis that is truly false. Preliminary analyses leading to a priori hypotheses suggest that differences noted are less likely to be from chance alone. Differences between groups for these hypotheses on multiple related outcome measures would support the treatment effect of NAC on cannabis use. Thus, we specified, a priori, the primary outcome as well as secondary comparisons and did not adjust for multiple comparisons of groups on these outcomes (
35–
37). All statistical analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, N.C.). The significance threshold was set at a p value of 0.05 (two-sided).
Results
Participants were enrolled between September 2009 and January 2011. A total of 136 adolescents were assessed for eligibility; of these, 20 (15%) were excluded because they did not meet eligibility criteria (see the figure in the data supplement that accompanies the online edition of this article). Baseline demographic and clinical characteristics are presented in
Table 1. The randomized cohort (N=116) was an older adolescent sample (mean age, 18.9 years [range=15–21]) and was predominantly white (N=96, 83.5%). There were no significant between-group differences in baseline demographic or clinical variables. The groups had similar rates of positive urine cannabinoid tests at baseline.
Efficacy
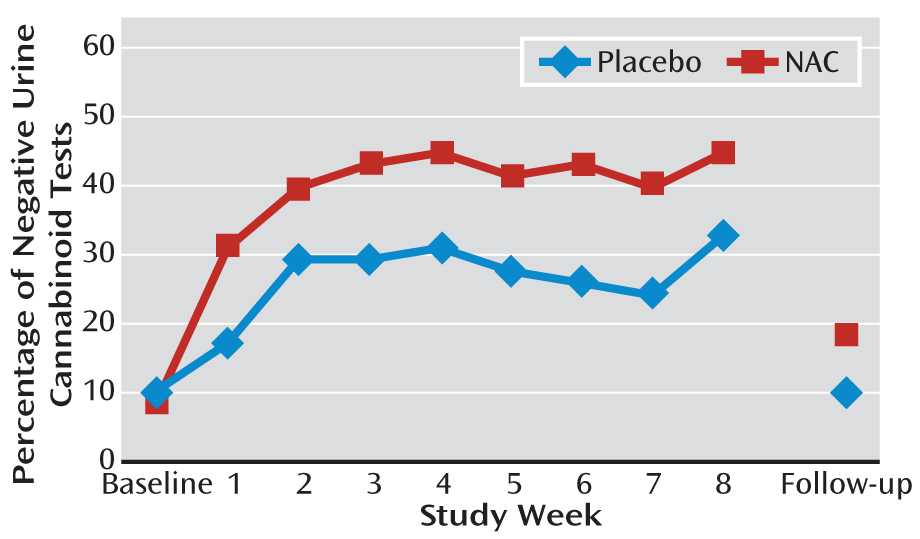
The proportion of negative urine cannabinoid tests in the NAC and placebo groups at each visit (intent-to-treat sample) is illustrated in
Figure 1. Although there were no group differences in baseline years of cannabis use (p=0.72) or presence of major depressive disorder (p=0.16), these variables were independent predictors of positive urine cannabinoid tests during treatment (p=0.007 and p=0.066, respectively) and therefore were covaried in the primary model along with baseline urine cannabinoid test result. Participants in the NAC group had more than double the odds of negative urine cannabinoid tests during treatment compared with those in the placebo group. In the adjusted model, a significant relationship was observed between treatment and the odds of a negative urine cannabinoid test (odds ratio=2.4, 95% CI=1.1–5.2; χ
2=4.72, p=0.029). There was no significant differential drug effect over time (treatment-by-time interaction). Through the final treatment visit, 40.9% (190/464) of the urine cannabinoid tests in the NAC group were negative, compared with 27.2% (126/464) in the placebo group, per intent-to-treat analysis, assuming any missing urine test was positive for cannabinoids. At the posttreatment follow-up visit, 19.0% (11/58) of the urine cannabinoid tests in the NAC group were negative, compared with 10.3% (6/58) in the placebo group. While still numerically favoring NAC, the overall treatment effect lost statistical significance at the posttreatment follow-up (adjusted odds ratio=2.4, 95% CI: 0.8–7.5; χ
2=2.2, p=0.131).
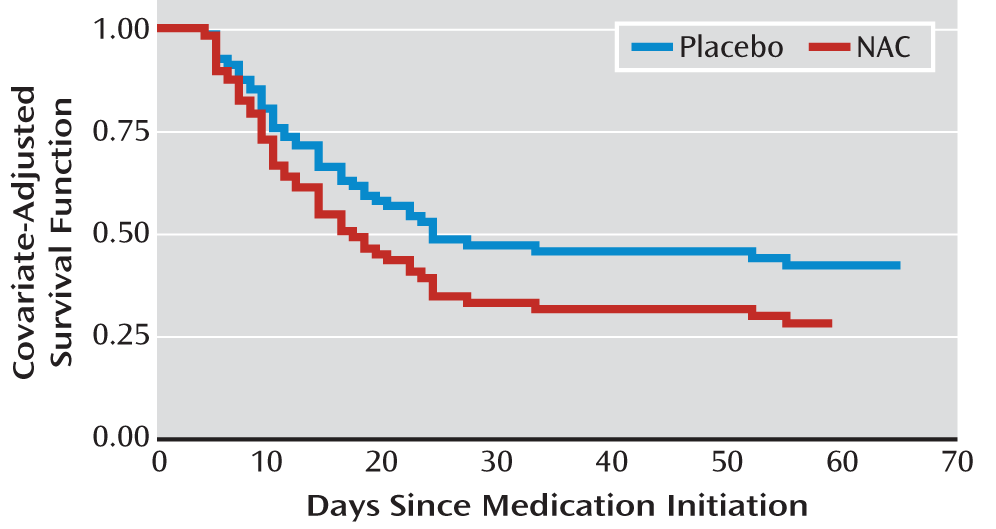
The secondary efficacy measures, assessing time to first negative urine cannabinoid test (hazard ratio=1.5, 95% CI=0.9–2.5; χ
2=2.1, p=0.146) (
Figure 2) and end-of-treatment abstinence (odds ratio=2.3, 95% CI=1.0–5.4; χ
2=3.7, p=0.054), revealed a similar magnitude of estimates favoring NAC, although the study was not adequately powered to assess these outcomes (
Table 2). There was no significant difference in percentage of self-reported days of cannabis use throughout treatment.
In the adjusted primary analysis model, study week and the treatment-by-study week interaction were not significant. However, participants with a negative baseline urine cannabinoid test had nearly six times the odds of negative tests during treatment (odds ratio=5.9, 95% CI=2.0–17.7; χ2=5.4, p=0.020). Similarly, those with fewer baseline years of cannabis use had significantly greater odds of negative urine tests during the study (odds ratio=1.4, 95% CI=1.1–1.7; χ2=8.0, p=0.047). Participants with major depressive disorder had lower odds of negative urine test during treatment, although this relationship fell short of statistical significance (odds ratio=0.3; 95% CI=0.1–1.0; χ2=3.5, p=0.062). Models were additionally examined for possible confounding and effect modification of age, weight, gender, race, psychiatric comorbidities, and number of previous cannabis quit attempts, revealing no significant confounders or effect modifiers.
Based on the intent-to-treat sample, with missing urine samples assumed to be positive for cannabinoids, the number needed to treat to achieve negative cannabinoid testing was 7.3 for the treatment portion of the study and 11.6 for the posttreatment follow-up visit. These are comparable to numbers needed to treat for several established addiction-targeted pharmacotherapies (
38).
A post hoc sensitivity analysis was performed on proportion of negative urine cannabinoid tests during treatment, using multiple methods to manage missing data and dropout. In addition to the intent-to-treat approach noted above (N=116), a modified intent-to-treat analysis that examined participants who received at least one dose of study medication (N=106) and a per-protocol analysis using available data (varying Ns) were performed. Using a modified intent-to-treat analysis, participants in the NAC group had 2.1 times the odds of having negative urine cannabinoid tests during treatment compared with those in the placebo group (adjusted odds ratio=2.1, 95% CI=1.0–4.5; χ
2=4.0, p=0.047). When examining only available data (per-protocol analysis), participants in the NAC group had 2.4 times the odds of having negative urine cannabinoid tests compared with those in the placebo group (adjusted odds ratio=2.4, 95% CI=1.1–5.4; χ
2=4.4, p=0.036). Finally, combinatorial graphical methods for assessing the impact of missing data on the significance of findings were also employed, in which every permutation of missing data assignment was considered, and a subsequent logistic regression was performed (
39). For the majority of missing data assignments that could be reasonably expected, the odds ratio remained significant. In general, the selection of missing data handling method had little effect on analytic outcomes.
Safety and Tolerability
Interim monitoring of adverse events was conducted every 6 months by an independent data and safety monitoring board. There were no FDA-defined serious adverse events, and there were no significant differences between the two treatment groups in the occurrence of any adverse events (38 events in the NAC group [in 24 participants] and 46 events in the placebo group [in 27 participants]). The most common adverse event was upper respiratory infection, which occurred in 19 participants (11 in the NAC group and eight in the placebo group). Adverse events occurring in at least two participants and deemed at least possibly treatment related included vivid dreams (three in the NAC group), insomnia (three in the placebo group), and irritability (two in the placebo group). One participant in the NAC group discontinued medication treatment because of severe heartburn, which resolved on discontinuation. No other participants in either group discontinued medication because of adverse events.
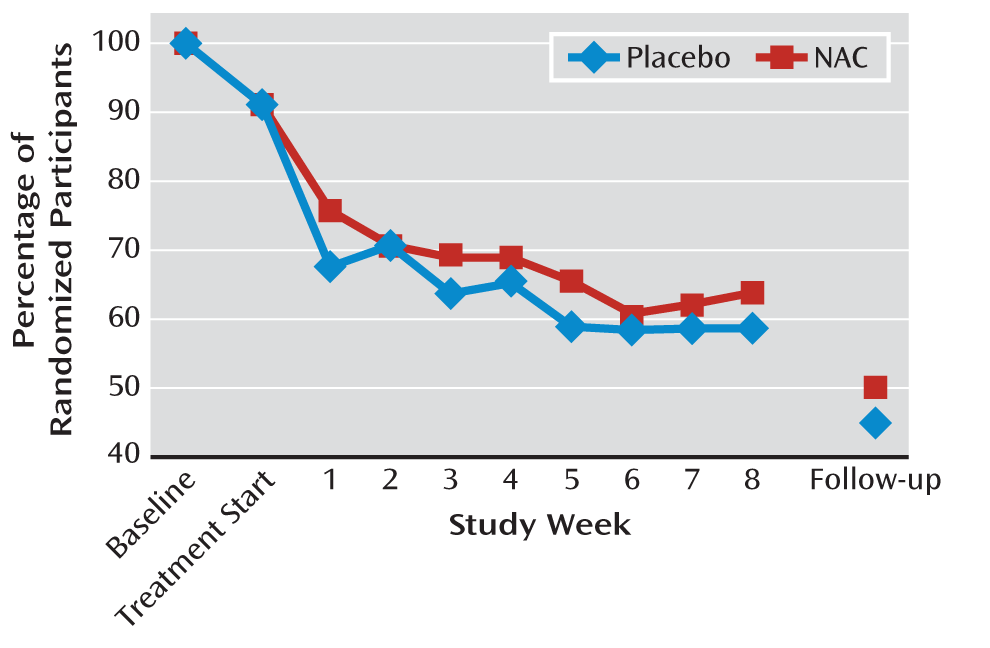
Retention and Adherence
Of the 116 randomized participants, 106 (92%) took at least one dose of study medication, 70 (60%) were retained through completion of treatment, and 54 (47%) were retained through posttreatment follow-up (
Figure 3). There was no significant between-group difference in retention to treatment completion (37 [64%] in the NAC group and 33 [57%] in the placebo group) or to posttreatment follow-up (29 [50%] and 25 [43%], respectively). Time to dropout, assessed using Cox proportional hazards regression models, was not significantly different between treatment groups. The median number of days retained in the study was 63 days (interquartile range, 13–66) in the NAC group and 62 days (interquartile range, 17–65) in the placebo group. Time to dropout was not significantly associated with any of the demographic or clinical characteristics. Review of medication diaries and weekly pill counts indicated that 95% of dispensed NAC doses and 93% of dispensed placebo doses were taken. Via contingency management procedures, participants in the NAC group earned $162 (SD=129) (of a possible $320) for adherence and $86 (SD=106) (of a possible $320) for abstinence (total=$248, SD=214), and those in the placebo group earned $141 (SD=117) for adherence and $54 (SD=98) for abstinence (total=$199, SD=190).
Discussion
To our knowledge, this is the first double-blind randomized placebo-controlled trial of pharmacotherapy for cannabis dependence in any age group yielding a positive primary cessation outcome via intent-to-treat analysis. The results support the hypothesis that treatment with NAC, compared with placebo, when added to contingency management and brief cessation counseling, yields improved cannabis abstinence during treatment. NAC more than doubled the odds of having negative urine cannabinoid tests as compared with placebo, and differences were detectable within a week of treatment initiation. Exploratory secondary abstinence outcomes numerically favored NAC but were not statistically significant. NAC was well tolerated, supporting its safety in cannabis-dependent adolescents. Given the increasing prevalence and adverse consequences of adolescent cannabis use and the limited abstinence rates produced by existing treatments, these findings provide an important addition to the evidence base.
While NAC, via its reversal of drug-induced glutamate dysregulation, has demonstrated significant effects on drug seeking and self-administration in animal models and has been the subject of encouraging preliminary human studies, this is the first randomized trial to demonstrate significant main effects of this agent on substance use cessation. This successful translational effort is particularly notable considering that no preclinical NAC studies focused on cannabis or cannabinoid administration. It appears that the neurobiological and behavioral effects of NAC may not be specific to a particular substance, suggesting that NAC may be a promising candidate medication for treatment of other substance use disorders, whether by modulation of glutamate or by other mechanisms.
This study incorporated contingency management, arguably the most efficacious youth-targeted psychosocial cannabis cessation treatment, which could have created a cessation “ceiling effect” and diminished the opportunity to detect an added medication effect. The finding that coadministration of NAC significantly increased abstinence even in this context is striking. It is thus tempting to argue that, by extension, NAC would enhance cessation outcomes on its own or when added to other psychosocial treatments. However, these possibilities can only be addressed by further controlled trials of NAC in other treatment contexts. It may be that NAC and contingency management exert synergistic treatment effects, a possibility that can be investigated in a 2×2 trial (NAC versus placebo and contingency management versus noncontingency management) to detect interaction effects (
40). Alternatively, it may be that NAC requires a nonspecific but powerful psychosocial treatment platform to exert its effects.
Our findings should be interpreted in light of the study's limitations. We investigated only one NAC dosing regimen over only 8 weeks, and the study was conducted at a single university-based research clinic with a relatively small sample of older adolescents within a narrow age range who presented with low rates of psychiatric comorbidity. The study was not powered to detect end-of-treatment abstinence or sustained posttreatment effects, or designed to evaluate potential effect mediators, such as NAC-induced changes in psychiatric symptoms. Additional research is needed to replicate these findings in other settings and to explore the efficacy of NAC at varying doses, across different age groups, with longer treatment duration and posttreatment follow-up, with a direct test of the integrity of the blind, and with more stringent efficacy measures (e.g., sustained abstinence at end of treatment). Additionally, as noted above, to be optimally implemented as a viable treatment, NAC must be investigated in a variety of psychosocial treatment contexts. While NAC's over-the-counter availability, low cost, and established safety profile make it highly desirable for eventual dissemination, these characteristics may prompt patients or providers to prematurely consider NAC as a standalone treatment. Nonetheless, our findings in this study represent a key step in the development of inexpensive, readily available, safe, and efficacious pharmacotherapy for cannabis dependence and should serve as a springboard for further investigation.
Acknowledgments
The authors thank the adolescents and families who participated in the study and acknowledge the tremendous contributions of the clinical research team, including Sarah Farber, Jessica Lydiard, and Christine Horne.