Children with conduct problems are at risk of developing persistent antisocial behavior and other mental and physical health problems (
1,
2). Conduct problems are a common reason for a referral to mental health and educational services and represent a considerable public health cost (
3). Callous-unemotional traits (lack of empathy and guilt, shallow affect) characterize a particularly problematic group of children with more severe conduct problems (
2). Twin studies suggest that conduct problems with callous-unemotional traits are highly heritable, while conduct problems without callous-unemotional traits are driven primarily by environmental influences (
4,
5). Children with conduct problems and high callous-unemotional traits are characterized by deficits in the processing of others’ fearful and sad facial expressions and vocal tones (
2,
6). In contrast, those with low callous-unemotional traits appear oversensitive to perceived social threat, including anger and even ambiguous neutral expressions (
2,
7). Inclusion of callous-unemotional traits as a conduct disorder specifier is being considered for DSM-5 (
8).
Most functional MRI (fMRI) studies of children and adolescents with conduct problems have reported atypical activation of the amygdala (
9,
10), a subcortical structure implicated in the processing of salient stimuli, including emotional facial expressions (
11). fMRI data focusing on children with conduct problems without accounting for individual differences in callous-unemotional traits have been mixed, with evidence of both amygdala hypoactivity and hyperactivity to affective stimuli (
12–
14). These mixed findings may partly reflect differences in paradigms used across studies. They may also partly reflect variations in callous-unemotional traits across samples, given significant differences in emotional reactivity and behavioral responses to emotional stimuli in children with high compared with low callous-unemotional traits (
2,
7).
Lower amygdala activity to fearful facial expressions has been reported in children with conduct problems and high callous-unemotional traits compared with typically developing children or children with symptoms of attention deficit-hyperactivity disorder (ADHD) (
15,
16). A recent study from our group measured fMRI responses in children with conduct problems while they watched scenarios requiring affective (versus cognitive) theory of mind (i.e., the ability to understand emotions as opposed to intentions and beliefs). Unique variance associated with callous-unemotional traits was negatively related, while unique variance associated with conduct disorder symptoms was positively related to amygdala response in children with conduct problems (
17). This finding mirrors behavioral studies documenting deficits in the processing of fear and sadness in children with conduct problems and high callous-unemotional traits (
6) but heightened sensitivity to social threat in those with low callous-unemotional traits (
2,
7), and it further suggests heterogeneity in amygdala reactivity to emotional stimuli in children with conduct problems. Amygdala hypoactivity in children with conduct problems and high callous-unemotional traits (
15–
17) could partly explain associated clinical phenomena such as premeditated aggression, lack of empathy, and difficulty in learning from punishment (
18). In contrast, amygdala hyperactivity in children with conduct problems and low callous-unemotional traits (
12,
17) may partly explain clinical phenomena such as reactive aggression and difficulty in regulating emotions (
2).
The amygdala responds to salient stimuli both when stimuli are presented preattentively (i.e., before reaching conscious awareness or attention [
19]) and under prolonged viewing conditions (
11,
20,
21). This is consistent with the amygdala’s role as part of a functional network engaged in triggering an orienting response to salient stimuli, including emotional facial expressions, so that appropriate processing of and behavioral responses to such stimuli can be prioritized. To date, fMRI studies of children with conduct problems have focused on affective stimuli presented only under prolonged viewing conditions. However, atypical amygdala response to preattentively presented affective stimuli may also characterize some children with conduct problems.
A recent behavioral study by Sylvers et al. (
22) assessed time taken for emotional faces to break through to conscious awareness during a continuous flash suppression task. Elevated callous-unemotional trait scores were associated with greater lag times for fearful, and, to a lesser extent, disgusted faces, to break through to conscious awareness relative to neutral faces. This effect was particularly pronounced in children with high levels of impulsive behavior. These preattentive data complement studies showing a fear processing deficit to overtly presented stimuli in children with conduct problems and high callous-unemotional traits.
To our knowledge, the present study is the first to use fMRI to investigate preattentive fear processing in children with conduct problems. We focused on fear processing as fearful faces signal potential threat in the surroundings and index distress. Children with conduct problems and high callous-unemotional traits are fearless and insensitive to others’ distress (
2). In contrast, children with conduct problems and low callous-unemotional traits are emotionally reactive to threat (
2). Extrapolating from previous data, we predicted that children with conduct problems and high callous-unemotional traits would show the lowest amygdala response to preattentively presented fearful versus calm faces, children with conduct problems and low callous-unemotional traits would show the greatest response, and typically developing comparison children would show an intermediate response.
Method
Participants
Boys 10–16 years of age were recruited from the community through newspaper advertisements and from local schools. Screening questionnaires were administered to parents and teachers of 176 boys expressing an interest in taking part. The questionnaires provided research diagnoses of conduct problems, dimensional assessments of callous-unemotional traits, an overall psychopathology screen, demographic data, and information regarding neurological or psychiatric diagnoses. Current conduct problems were assessed using the conduct disorder subscale of the Child and Adolescent Symptom Inventory–4R (
23), and callous-unemotional traits were assessed using the Inventory of Callous-Unemotional Traits (
24). Both were scored by taking the highest ratings from either the parent or the teacher questionnaire for any given item (
25). The Strengths and Difficulties Questionnaire (
26) was used as a brief screening measure for psychopathology (see Table S1 in the
data supplement that accompanies the online edition of this article).
On the basis of the screening information, participants were invited for an fMRI scan; this group largely overlapped with a previous sample (
17). The cutoff conduct disorder subscore on the Child and Adolescent Symptom Inventory–4R for inclusion in the conduct problems group was 3 for boys 10–14 years of age and 6 for those 15–16 years of age; scores of these magnitudes and above are associated with a clinical diagnosis of conduct disorder (
27). Boys with conduct problems were divided into low and high callous-unemotional trait groups based on a median split of callous-unemotional trait scores (median=44.5).
Groups were matched on IQ, age, handedness, ethnicity, and socioeconomic status. All comparison subjects scored below the conduct problems group median on callous-unemotional traits and scored in the normal range on each subscale (including conduct disorder) of the Strengths and Difficulties Questionnaire. For all groups, exclusion criteria included a previous diagnosis of a neurological or psychotic disorder or a current prescription for a psychiatric medication. (We later learned that two participants had been on medication for ADHD symptoms at the time of scanning. However, analyses conducted with and without these participants were very similar, so their data are included in reported analyses). To ensure that we had a representative group of boys with conduct problems, common comorbidities (ADHD, generalized anxiety disorder, depression, and substance abuse) were not used as exclusion criteria, but current parent-reported symptom counts were obtained during fMRI sessions using the Child and Adolescent Symptom Inventory–4R so that any contribution to the imaging data could be systematically assessed.
After parents and children received a complete description of the study, parents provided written informed consent and participants provided assent. Fifty-five boys underwent scanning (38 in the conduct problems groups, 17 in the comparison group), yielding a final sample of usable data from 30 boys with conduct problems (15 in each callous-unemotional group) and 16 comparison subjects. Exclusions were due to excessive motion (five in the conduct problems group and one in the comparison group), refusal to undergo scanning (two in the conduct problems group), and technical problems (one in the conduct problems group). Group assignment based on callous-unemotional traits took place after exclusions, based on the median for the final sample. Demographic and clinical data for participants are summarized in
Table 1.
Experimental Task
The task was based on backward masking methods that have been used in previous studies to elicit amygdala response to preattentively presented stimuli in healthy adults (
21,
28). Stimuli comprised fearful and calm faces of six individuals (three male, three female) taken from the NimStim Set of Facial Expressions (
29). Calm (not neutral) faces were used, as previous studies suggest that children with conduct problems may interpret neutral faces as hostile (
7). Image size was standardized, and all faces were presented in grayscale with hair cropped. Stimuli were presented on a midgray background in 20 blocks, 10 fear and 10 calm, each lasting 15 seconds. Block order was randomized, with the constraint that the same block type was never presented more than twice in a row. A fixation cross was displayed for 15 seconds after every second block.
Each block consisted of 30 trials comprising a target face presented for 17 ms, followed by a backward mask face presented for 183 ms. The subjective experience is of seeing the backward masked face only, with the target face presented below the level of conscious awareness (preattentively). A crosshair interstimulus interval was presented for 300 ms at the center of the screen, with the center of the cross approximating the center of the nose of the target and mask faces. Each trial lasted 500 ms. The only difference between fear and calm blocks was that target (masked) faces were either fearful or calm. All mask stimuli were calm faces. Presentation of the target face for one frame (17 ms) was verified with a high-speed video camera set to capture 1,000 frames per second.
For each block, the 30 trials comprised five presentations of each of the six target faces in a pseudorandom order, with each target face (fearful or calm) masked by each of the other five individuals’ calm faces. The task lasted 7.5 minutes and comprised 600 trials (300 fear, 300 calm). Participants were asked to keep their eyes fixed on the central cross during the task and to attend to the faces (passive viewing). Participants were monitored by video to ensure alertness. Afterward, participants were asked what they had seen. Three participants mentioned seeing emotion, although none explicitly mentioned fear. Removing these participants from the analysis did not alter the results, and their data were retained in the final sample.
Psychometric and Questionnaire Measures
Participants completed the two-subtest version of the Wechsler Abbreviated Scale of Intelligence (
30) as well as the Alcohol Use Disorder Identification Test and the Drug Use Disorder Identification Test (
31,
32). In addition, the Child and Adolescent Symptom Inventory–4R scales for ADHD, generalized anxiety disorder, and major depressive episode were completed by a parent or guardian to ascertain symptom counts for disorders most commonly comorbid with conduct problems (
Table 1). Group differences were observed for all symptoms and were controlled for in subsidiary analyses. Symptoms were not included as covariates in the main analysis, because a strong case has been made that when participants are not randomly assigned to groups, it is inappropriate to covary for variables intrinsically related to group assignment (
33).
fMRI Data Acquisition
A Siemens Avanto 1.5-T MRI scanner was used to acquire a 5.5-minute three-dimensional T
1-weighted structural scan and multislice T
2*-weighted echo planar volumes with blood-oxygen-level-dependent contrast. The echo planar imaging sequence was designed to optimize signal detection and reduce dropout in the orbitofrontal cortex and amygdala (
34), and it used the following acquisition parameters: 35 2-mm slices acquired in an ascending trajectory with a 1-mm gap; TE=50 ms; TR=2975 ms; slice tilt=−30° (T>C); flip angle=90°; field of view=192 mm; matrix size=64×64. Functional data were acquired in a single run of 7.5 minutes, with 158 volumes per run. Field maps (phase and magnitude images) were also acquired for use in the unwarping stage of data preprocessing.
fMRI Data Analysis
Imaging data were analyzed using SPM8 (
www.fil.ion.ucl.ac.uk/spm). Data preprocessing followed a standard sequence: the first five volumes were discarded, and the data were realigned, unwarped using a field map, normalized with a voxel size of 2×2×2 mm, and smoothed with an 8-mm Gaussian filter. A block analysis compared neural responses associated with masked fearful and calm faces. Three regressors, each comprising 10 15-second blocks of fear, calm, and fixation, were modeled as boxcar functions convolved with a canonical hemodynamic response function. The six realignment parameters were modeled as effects of no interest. For 10 participants (two in the comparison group and four in each conduct problems group), an extra regressor was included to model a small number of corrupted images resulting from excessive motion. These images (≤10% of each participant’s data) were removed and the adjacent images interpolated in order to prevent distortion of the between-subjects mask. Data were high-pass filtered at 128 seconds to remove low-frequency drifts.
First-level contrast images for fear-calm for each participant were entered into second-level analyses. Based on our prediction that amygdala responses to fear-calm would vary by group, a regression analysis was conducted with groups coded as 1, 0, and −1 (1=group with conduct problems with low callous-unemotional traits; 0=comparison group; −1=group with conduct problems with high callous-unemotional traits). A t-contrast of 1 was used to look for regions showing a linear relationship across groups in the predicted direction, and −1 for the reverse direction. To explore dimensional associations between callous-unemotional traits and amygdala response to masked fear within the conduct problems group, an additional regression analysis was conducted in which individuals’ callous-unemotional trait scores were regressed against neural responses to fear-calm.
We report data from the amygdala region of interest in the main text; for completeness, we also report results from whole brain analyses at p<0.001, uncorrected, k≥5 in the online
data supplement. The amygdala region of interest was defined both structurally (the bilateral Talairach Daemon amygdala mask supplied by the Wake Forest University PickAtlas (
35) and functionally (an 8-mm sphere centered on the peak coordinate [x=18, y=−6, z=−18] for masked fear > masked happy [
21], converted from Talairach to Montreal Neurological Institute coordinates).
Results
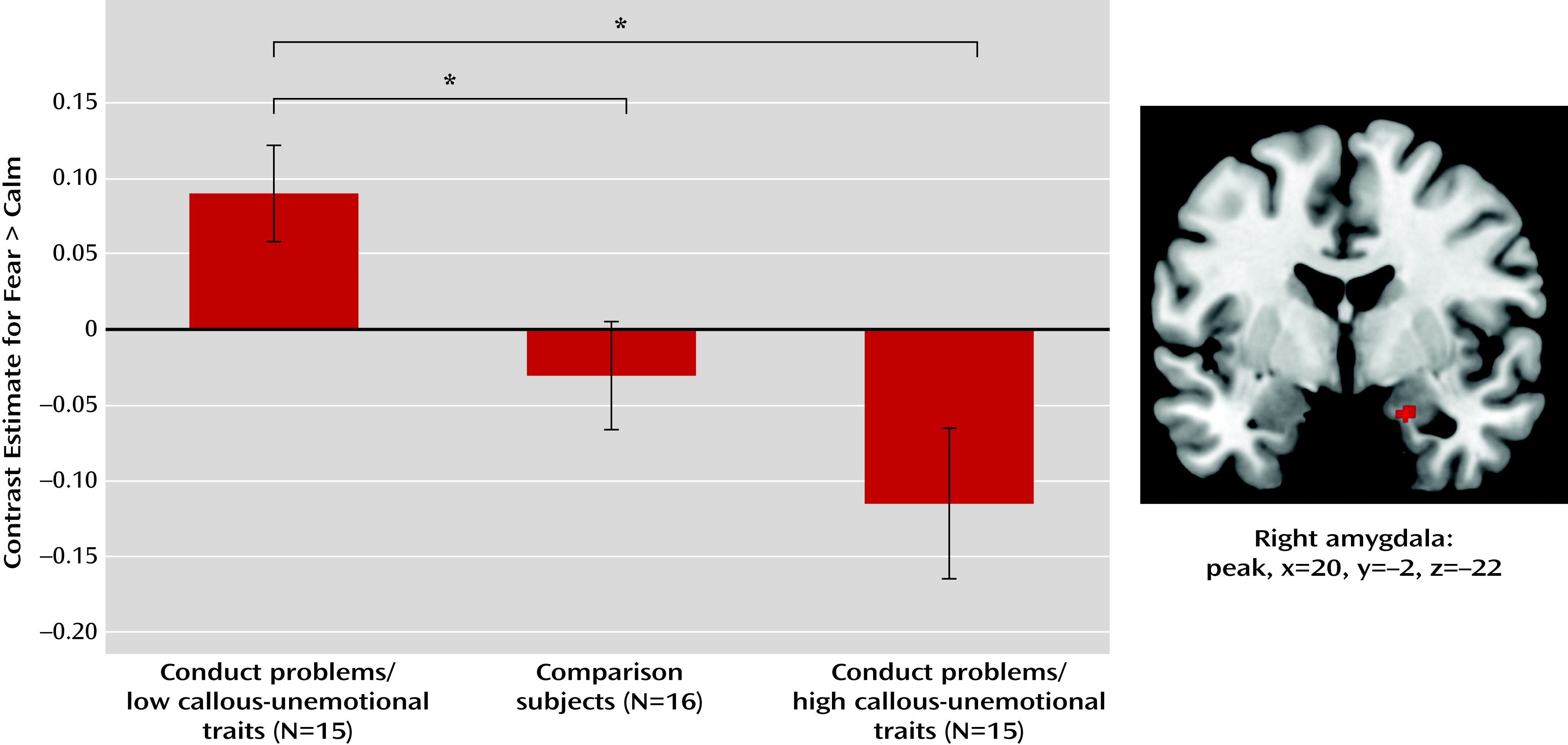
A cluster showing the predicted pattern (conduct problems with low callous-unemotional traits > comparison subjects > conduct problems with high callous-unemotional traits) was found in the right amygdala at p<0.001, uncorrected (peak voxel: x=20, y=−2, z=−22; t=3.85, z=3.55, k=9) (
Figure 1). The whole cluster survived small-volume correction using both the structurally and functionally defined amygdala regions of interest (p<0.05, family-wise error corrected at both voxel and cluster levels). This finding also remained significant with family-wise error correction when controlling for variables on which the groups differed (conduct disorder, ADHD, anxiety, and depression symptoms; see Table S2 in the online
data supplement). For completeness, a list of all clusters showing the predicted pattern for fear-calm and the reverse is presented in Table S3 in the
data supplement. As no regions survived whole-brain correction, these data are not discussed further.
Planned t tests were conducted using mean responses across the right amygdala cluster. One-sample t tests comparing responses to fear-calm in each group revealed a significant positive difference in boys with conduct problems and low callous-unemotional traits (t=2.82, df=14, p=0.014), no significant difference between conditions in comparison boys, and a significant negative difference in boys with conduct problems and high callous-unemotional traits (t=−2.30, df=14, p=0.037). Across groups, responses to fear-calm in boys with conduct problems and low callous-unemotional traits were significantly greater than in both comparison boys (t=2.49, df=29, p=0.019) and boys with conduct problems and high callous-unemotional traits (t=3.46, df=28, p=0.002). The difference between the comparison group and the group with conduct problems and high callous-unemotional traits was not significant.
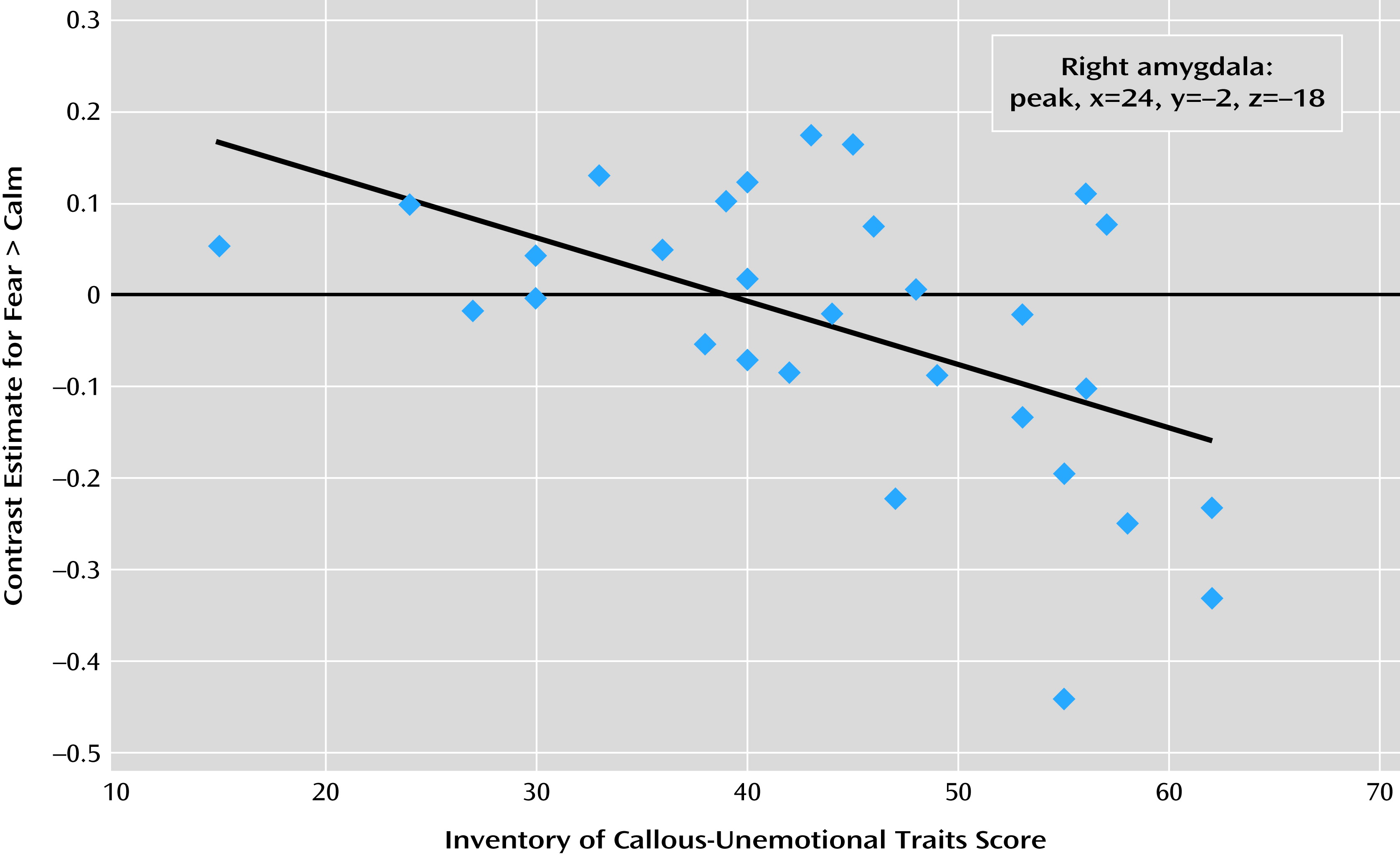
A separate regression analysis within the conduct problems group was conducted to investigate the association between dimensional callous-unemotional trait scores and neural responses to preattentively presented fear (relative to calm). At a whole-brain uncorrected threshold of p=0.001, one voxel in the right amygdala showed a significant negative relationship with callous-unemotional trait scores (x=24, y=−2, z=−18; t=3.38, z=3.07) (
Figure 2). This voxel also survived small-volume correction (p<0.05 with family-wise error correction) using the regions of interest defined both anatomically and functionally as described above. Other regions showing negative or positive relationships with callous-unemotional trait scores are listed in Table S4 in the online
data supplement. None survived whole-brain correction, and these data are not discussed further.
Discussion
Using a backward masking paradigm (
21) to investigate differential amygdala response to preattentively presented fear in boys with conduct problems, we found significantly lower amygdala activity to backwardly masked fearful versus calm faces in boys with high compared with low callous-unemotional traits. The amygdala activity level in the comparison group was intermediate between those of the conduct problems groups. To our knowledge, these data are the first to demonstrate differential amygdala activity to preattentively presented fear across the spectrum of callous-unemotional traits in children with conduct problems. Our findings indicate that reduced amygdala activation to salient stimuli in children with conduct problems and high callous-unemotional traits extends to early stages of information processing, suggestive of an affective processing deficit in this group. Reduced amygdala activation is characteristic of this subgroup rather than of children with conduct problems more generally. Our finding adds to increasing evidence regarding the utility of a callous-unemotional specifier in the classification of children with conduct problems.
In a previous study (
17), we reported a negative association between callous-unemotional traits and amygdala activity using an explicit and complex affective processing task. The present study demonstrated a negative association between callous-unemotional traits and amygdala response to preattentively presented fear, further highlighting the dimensional relationship between callous-unemotional traits and amygdala activity. This finding is consistent with a recent behavioral study demonstrating reduced preattentive processing of negative emotions in individuals with high callous-unemotional traits (
22).
Lesion studies indicate an important role for the amygdala in at least some aspects of preattentive processing of salient stimuli (see reference
11 for a review). For example, reduced reflexive gaze orientation to fearfully widened eyes is seen in patients with amygdala damage (
36). It has been proposed that amygdala dysfunction may interfere with the initial processing of salient facial features (e.g., widened fearful eyes) that typically trigger attentional shifts (
36,
37). Studies of healthy adults have found that displays of fearful eye whites are sufficient to elicit amygdala activation (
38) and that extent of amygdala activation to emotional faces is correlated with degree of fixation to the eye region (
37). Our data suggest that children with conduct problems and high callous-unemotional traits show lower amygdala activity to preattentively presented salient facial information, which could compromise their orienting to critical affective cues relevant for social interaction. Recent behavioral and eye-tracking data are consistent with this possibility (
7,
39). Under free-viewing conditions, children with high callous-unemotional traits have difficulty recognizing fearful expressions and focus less on the eye region of the face than children with low callous-unemotional traits. However, when asked to effortfully focus on the eye region of the face, fear recognition performance improves.
It is important to acknowledge that the amygdala is part of a network that triggers an orienting response (
37). Future studies using paradigms that are designed to explore functional connectivity between the amygdala and other brain regions will be informative. We also note that our comparison group did not show increased amygdala response to preattentively presented fear. Some studies using masked stimuli have also failed to find robust amygdala response in healthy adults (
40), suggesting that there may be individual differences in response to preattentively presented stimuli. In the present study, the task was sensitive enough to elicit differences between conduct problem subgroups based on callous-unemotional traits. Additionally, our group difference finding was right-lateralized, in line with most previous effects reported for masked fear stimuli (
40).
Several limitations of this study must be acknowledged. Our sample was selected using a research diagnosis: replication in a sample of youths with a clinical diagnosis would be of interest. In addition, we studied only boys; it is unknown whether girls with conduct problems show a similar pattern. Because we used a passive viewing task, it was not possible to delineate specific computations contributing to activation differences between groups. Future imaging studies using more temporally sensitive methods could explore the time course of amygdala activity and connectivity with other brain regions. Finally, this study was cross-sectional. Longitudinal studies are needed to chart possible changes in patterns of neural responses associated with conduct problems.
Despite these limitations, this study extends our understanding of the neural correlates of conduct problems. We showed that right amygdala responses to preattentively presented fear differentiate children with conduct problems and high or low levels of callous-unemotional traits, with significantly greater responses in those with low levels. This mirrors findings from studies using explicitly presented affective stimuli and additionally suggests that altered amygdala responses characterize the earliest stages of affective processing. In children with conduct problems and high callous-unemotional traits, an attenuated amygdala response to preattentive fearful faces may reduce orienting to salient features of these stimuli, reducing opportunities to learn from these important social cues. Conversely, heightened preattentive amygdala responses in children with conduct problems and low callous-unemotional traits may predispose these children to affective hypervigilance. Our regression analysis also contributes to existing data indicating a dimensional relationship between callous-unemotional traits and amygdala response.
From a clinical perspective, divergent patterns of amygdala response to preattentively presented emotion point to differential underlying neural vulnerabilities in conduct problem subgroups. This may have important implications for how we formulate and intervene in conduct disorder. Specifically, it may be important to evaluate the therapeutic efficacy of helping children with conduct problems develop a more balanced appraisal of other people’s emotions. This may include a process of explicit verbalization. This kind of approach has already been shown to be effective in attention bias modification treatment for anxiety disorder (
41). In addition, clinically focused intervention studies that investigate how treatment response is related to a child’s level of callous-unemotional traits will help us better understand the variation in treatment response seen in this group.
Acknowledgments
The authors thank Dr. Oliver Josephs for technical advice and Mr. Philip Kelly, Ms. Elizabeth O’Nions, and Ms. Zoe Hyde for help with data collection and checking.
Drs. Viding and Sebastian contributed equally to this article.