The occurrence and severity of TBI are difficult to ascertain in veterans because of retrospective bias in determining relevant clinical variables, such as whether there was loss of consciousness or posttraumatic amnesia. Neuroimaging findings, however, hold promise in providing useful biomarkers for the presence of TBI.
The number of studies examining the DTI correlates of blast-related mild TBI is more limited. Mac Donald et al. recently (
12) examined diffusion abnormalities occurring in the early stages of mild TBI among soldiers who were evacuated from field operations. Comparing DTI parameters of 63 soldiers who had experienced mild TBI with those of 21 comparison soldiers who did not have a TBI, they observed that those with mild TBI showed reduced fractional anisotropy in the cerebellar peduncles, cingulate bundles, and orbitofrontal white matter relative to the comparison group. Furthermore, these changes appeared to be persistent in a subgroup (N=47) of patients who had a follow-up scan 6–12 months later. On the other hand, Levin et al. (
13) failed to identify DTI changes among veterans assessed a mean of about 2.4 years after mild TBI. They did, however, find correlations between fractional anisotropy values and neuropsychological performance.
These studies relied on region-of-interest and voxel-based analysis to identify white matter disruption. These methods assume that there is a spatial overlap of lesions across affected subjects, which is not consistent with the multifocal disruption of white matter pathways observed in human studies and experimental models of TBI (
14). Davenport et al. (
14) addressed this spatial heterogeneity of lesions by analyzing the distribution of fractional anisotropy values across total white matter voxels between Iraq and Afghanistan veterans with and without a history of mild TBI. The number of voxels with low fractional anisotropy (i.e., two or more standard deviations of the control group mean) was greater in the group of veterans exposed to blasts.
In the present study, we used DTI to examine white matter integrity in a relatively large group (N=72) of Iraq and Afghanistan veterans with a history of mild TBI. Veterans without a history of TBI (N=21) constituted the comparison group. We hypothesized that 1) mild TBI would be associated with a diffuse and heterogeneous pattern of injury; 2) DTI changes would be associated with severity of injury; 3) the presence of psychopathology (e.g., posttraumatic stress disorder [PTSD] and alcohol use disorders) would not have an effect on DTI findings; and 4) DTI abnormalities would be correlated with alterations in executive functioning.
Results
The demographic and clinical characteristics of the unexposed veterans, veterans with possible TBI, and veterans with probable TBI are summarized in
Table 1. Veterans with probable TBI had more education on average than those with possible TBI (Wilcoxon statistic=1,283.5, p=0.009). Time since returning from deployment was shorter on average for the veterans unexposed to TBI compared with time since trauma for the possible and probable TBI groups (Wilcoxon statistic=442, p=0.001, and Wilcoxon statistic=388, p=0.008, respectively). Participants with possible TBI had more blast episodes than the unexposed and probable TBI groups (Wilcoxon statistic=262.5, p<0.0001, and Wilcoxon statistic=1,234.5, p=0.04, respectively).
Voxel-Based Analysis
When controlling for multiple comparisons, tract-based spatial statistics analysis of the skeletonized white matter tracts revealed no differences in fractional anisotropy and mean diffusivity across the groups. These findings are consistent with those observed in previous studies of veterans of the Iraq and Afghanistan wars (
13).
Pothole Analysis
Taking into account that the negative findings obtained through voxel-based analysis can be attributed to the spatial heterogeneity of white matter lesions (
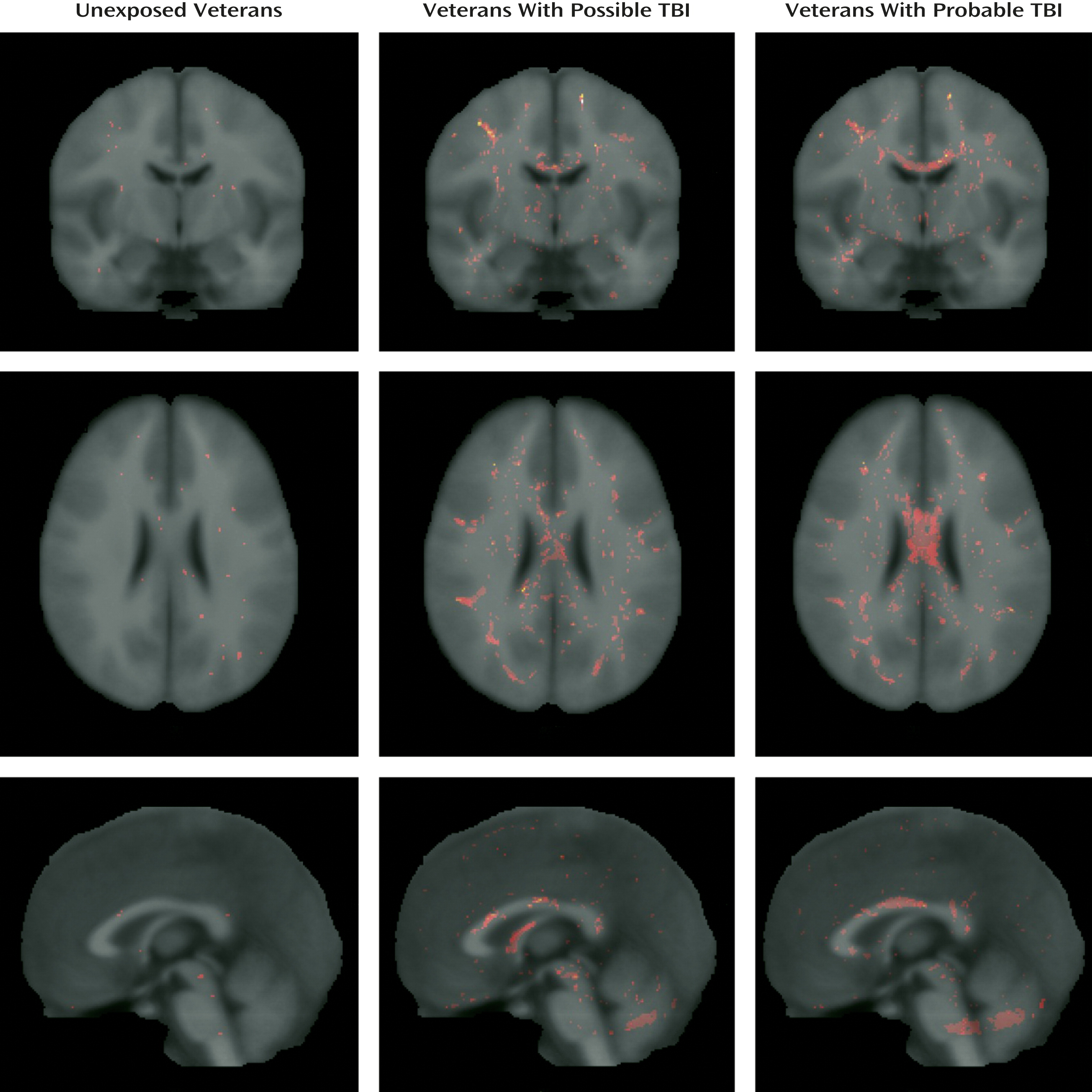
14), we compared the total number of potholes at a z-score threshold of –3 (
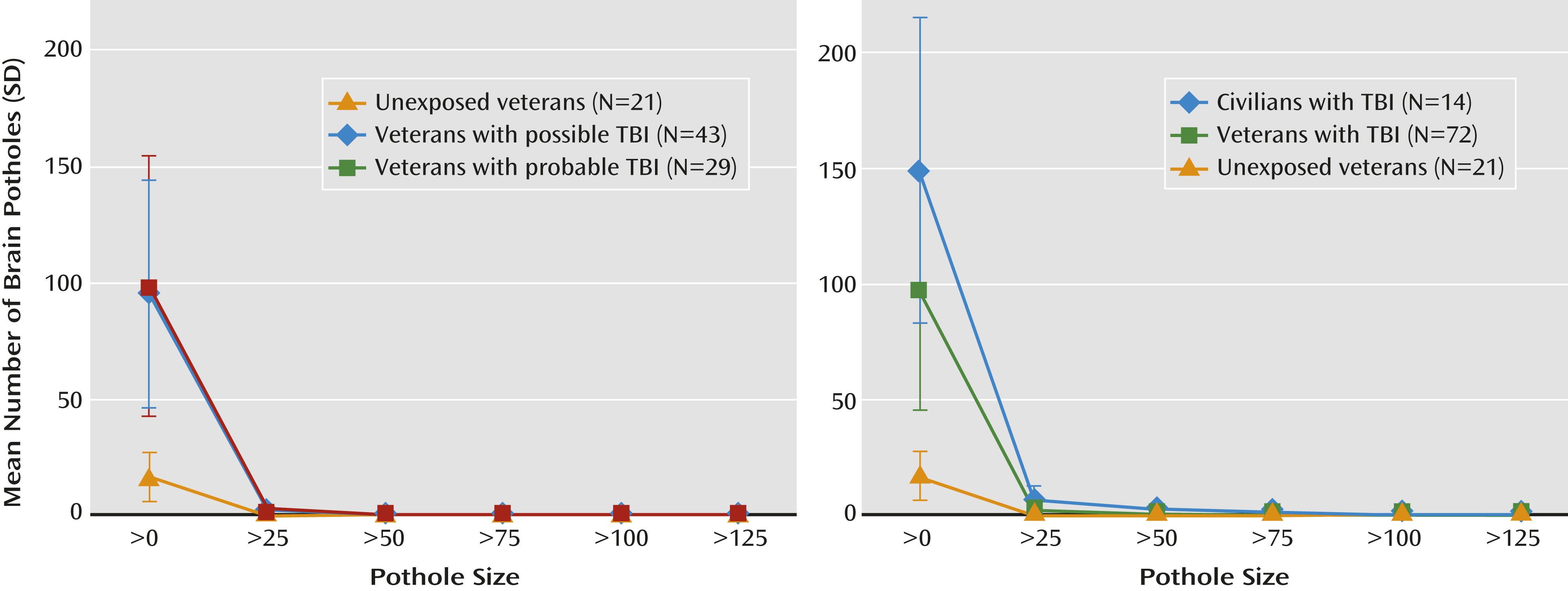
Figure 1). The three groups differed significantly in the total number of potholes (Kruskal-Wallis χ
2=46.2, df=2, p<0.0001). Specifically, the number of potholes was higher in veterans with probable TBI (mean=98.8, SD=56.1; Wilcoxon p<0.0001) and those with possible TBI (mean=95.3, SD=48.7) than the unexposed veterans (mean=16.8, SD=10.5; Wilcoxon p<0.0001) (
Figure 2).
To further investigate the differences in number of potholes between the three groups, we used a normal regression model with the log-transformed number of potholes as the dependent variable and TBI group and all the variables that differed between the groups as independent variables. Because the presence of PTSD, mood disturbance, and alcohol misuse might be associated with alterations in white matter tracts, we also investigated the effect of HAM-D, CAPS, and AUDIT scores on total number of potholes. After controlling for these possible confounders, the difference between groups remained significant (F=17.7, df=2, 80, p<0.0001). None of the possible confounders were significant predictors of the number of potholes. Excluding participants who had a TBI before deployment did not affect these results.
Once we had established an association between the presence of potholes and mild TBI, we examined whether similar white matter alterations could be observed in patients who experienced TBI in civilian settings.
Since we found no evidence of any difference in number of potholes between the possible and probable mild TBI groups, we collapsed the two groups into one (veterans with TBI, N=72) for further comparisons.
Table 2 summarizes the demographic and clinical characteristics of the unexposed veterans, veterans with TBI, and civilians with TBI.
The civilians with TBI were older on average than the veterans with TBI (Wilcoxon statistic=840.5, p<0.007) and had shorter time since trauma (Wilcoxon statistic=105, p<0.0001).
We found that total number of potholes was significantly different between the three groups (
Figure 1) (Kruskal-Wallis χ
2=54.3, df=2, p<0.0001). The civilian TBI group had more potholes than the veteran TBI and unexposed veteran groups (civilian TBI group, mean=148.6 [SD=66.0]; veteran TBI group, mean=96.7 [SD=51.4]; unexposed veteran group, mean=16.8 [SD=10.5]; Wilcoxon statistic=865, p=0.004, and Wilcoxon statistic=399, p<0.0001, respectively). Also, veterans with TBI had more potholes on average than unexposed veterans (Wilcoxon statistic=247.5, p<0.0001). After controlling for age, time since trauma, HAM-D score, and CAPS score, the difference between groups remained (F=70.5, df=2,89, p<0.0001). Excluding participants who had experienced a TBI before deployment or, in the case of civilians, before the current TBI did not affect these results.
We then examined whether number of potholes was associated with TBI severity, which we defined in terms of duration of posttraumatic amnesia using an ordinal scale (see the online
data supplement). Overall, number of potholes was positively associated with TBI severity (Spearman’s rho=0.58, p<0.0001, N=106). In addition, Glasgow Coma Scale scores (available only for the civilian group) were inversely associated with the total number of potholes (Spearman’s rho=–0.58, p=0.03, N=14). Furthermore, patients who experienced loss of consciousness at the time of the trauma had a greater number of potholes than those who did not (Wilcoxon statistic=2,360.5, p=0.0007, N=107).
Finally, to investigate the functional correlates of these white matter abnormalities, we examined whether number of potholes was associated with neuropsychological performance among the veteran groups. We observed a negative correlation between the number of potholes in the corpus callosum and performance in the executive domain (Spearman’s rho=–0.23, p=0.03). This association persisted when the analysis controlled for relevant variables such as age, education, time since trauma, and severity of PTSD symptoms.
Discussion
To our knowledge, this is the largest study in which DTI was used to examine the integrity of white matter pathways among veterans of the Iraq and Afghanistan wars who sustained mild TBI during their deployment. Voxel-based analysis did not demonstrate any differences between veterans with a history of mild TBI and those without exposure to TBI during deployment. However, several years after the trauma, veterans with a history of mild TBI had a higher number of diffusely distributed areas of decreased fractional anisotropy (potholes) than did veterans who did not experience TBI. Potholes were also observed in patients who experienced mild TBI in civilian settings and were examined within 90 days after the trauma. The difference between groups in number of potholes was not influenced by age, time since trauma, history of mild TBI unrelated to deployment, presence of mood or anxiety disorders, or antecedent alcohol misuse. Number of potholes was correlated with severity of TBI and with performance on executive functioning tasks.
The limitations of this study are related to the difficulty in making retrospective TBI diagnoses in veterans, particularly those with concurrent PTSD. It might be argued that selection of veterans without psychiatric comorbidity would reduce this variability. However, excluding subjects with these conditions would result in an unrepresentative sample of this population. In fact, time since trauma and coexisting psychopathology or alcohol misuse did not affect the results. Our findings, however, are applicable mainly to the group of symptomatic patients who are followed in polytrauma services in the VA system.
The failure to detect white matter abnormalities using voxel-based analysis replicates the findings of other studies of veterans of the Iraq and Afghanistan wars (
13,
14). The fact that DTI metrics were abnormal in the initial phase of mild TBI suggests the possibility that DTI abnormalities normalize with time. The available literature on civilian TBI shows that in the chronic stage, fractional anisotropy is persistently reduced in some white matter areas, while other areas show an increase in fractional anisotropy that is suggestive of axonal recovery. The extent of this regeneration process would influence the functional outcome of these patients (
35). This complex process of damage and repair probably also occurs in military mild TBI.
Discrepant voxel-based analysis findings in civilian and military samples may be related to the fact that the mechanism producing brain damage in blast injuries is different from the mechanisms involved in civilian TBI (
36). For instance, the vascular load produced by blast overpressure is an important etiological factor in blast injuries that plays no role in civilian injuries (
37,
38), while the inertial loadings that produce axonal injury in civilian TBI may be less important in blast TBI (
39). These biophysical differences may determine the size and spatial distribution of brain lesions as well as the timing of pathological events, such as the disruption of the blood-brain barrier and cerebral vasospasm (
36,
40,
41). For instance, axonal damage in blast injuries may be more marked in brainstem and cerebellar pathways (
12,
42).
In spite of the negative voxel-based analysis findings, veterans with mild TBI had a significantly greater number of potholes than those without TBI. The fact that potholes are observed more frequently in veterans with a history of mild TBI suggests that they are not an artifact resulting from the processing of DTI images. Consistently, potholes were observed with a greater frequency in a positive control group (civilians with mild TBI) than in a group of veterans with TBI or in a group of unexposed veterans. The incremental pattern in the number of potholes of these groups (
Figure 1) may be related to several factors. For instance, given that all the civilian participants with TBI were admitted to the hospital, they may have had more severe injuries. In addition, they were assessed earlier in their clinical course, when repair processes would not have been completed.
On the other hand, we did not find a difference in number of potholes between patients with probable TBI and those with possible TBI. This finding underscores the effect of retrospective bias in ascertaining TBI in the veteran population. Veterans often do not accurately recall the blast event and may overestimate or underestimate its consequences (
43). Although veterans in the possible TBI group experienced a greater number of blasts than those with probable TBI, we did not observe an effect of the number of injuries on the number of potholes that would suggest cumulative axonal pathology. Thus, we believe that retrospective bias is the major factor behind the lack of significant differences between the probable and possible TBI groups.
If potholes are related to traumatic axonal injury, we would expect a positive correlation between their number and the severity of TBI. We found that number of potholes was associated with a history of loss of consciousness and with duration of posttraumatic amnesia. Furthermore, we obtained similar results when analyzing Glasgow Coma Scale scores in the civilian group.
Finally, we found that the number of potholes in the corpus callosum was negatively correlated with performance in executive functioning tasks. DTI abnormalities of the corpus callosum have been consistently associated with the cognitive outcome of TBI (
31–
33,
44). Moreover, a recent study of veterans of the Iraq and Afghanistan wars found that soldiers with mild TBI had decreased interhemispheric coordination of brain activity, an effect that is probably related to damage to the anterior corpus callosum (
34). The association between number of potholes in the corpus callosum and performance on cognitive tasks remained significant independently of the effect attributable to the severity of PTSD symptoms, a major factor contributing to executive dysfunction among veterans of the Iraq and Afghanistan wars (
45).
White matter potholes may constitute a biomarker of axonal injury that can be identified in mild TBI at both the acute and chronic stages of its clinical course. They are associated with the severity of the injury and with relevant functional outcomes. Furthermore, pothole analysis may be more sensitive than conventional neuroimaging approaches given its ability to detect diffuse and spatially nonoverlapping defects. Future studies will need to replicate these findings in other groups of TBI patients with different etiologies and severity, delineate the longitudinal course of DTI abnormalities, and analyze the effect of repetitive mild TBI.