The study was performed at MAMC, a large Army medical facility located on Joint Base Lewis-McChord (JBLM), Washington, and at VA Puget Sound Health Care System (VAPS). It was approved by JBLM command, the local MAMC and VAPS institutional review boards, and the Department of Defense Clinical Investigation Regulatory Office. Soldiers provided written informed consent after reading the consent form and having an opportunity to ask questions.
Participants
Active-duty soldiers (N=65) and recently discharged Army veterans (N=2) who had served in the Iraq or Afghanistan conflicts were recruited through banners and posters stating, “Health Studies: Sleep Disturbance With Combat Nightmares” with a contact telephone number self-referral. Soldiers also were referred by MAMC and JBLM health care providers who were made aware of the study through briefings (N=34) by the investigators (M.A.R., T.W., K.P., J.C., J.H., J.O.) and by fellow soldiers who had completed the study. Soldiers were enrolled between December 2009 and April 2011. Study visits were carried out at a JBLM medical clinic for active-duty soldiers and at VAPS for veterans.
Potential participants were screened by telephone for the presence of recalled distressing combat-related nightmares at least 2 nights per week. Those who met this major inclusion criterion were provided detailed information about the study, including the 50% chance of receiving placebo and the fact that open-label prazosin treatment was available. Those who elected to participate in the study were given an appointment for informed consent and a detailed eligibility evaluation by VAPS research personnel embedded 2 days a week at JBLM. Recruitment flow is presented in Figure S1 in the
data supplement that accompanies the online edition of this article.
Inclusion and Exclusion Criteria
To be included in the study, participants had to meet DSM-IV criteria for PTSD; have a total score ≥50 on the 17-item CAPS; have a history of exposure to one or more life-threatening combat experiences that preceded onset of combat-related nightmares; have recalled distressing combat nightmares at least 2 nights/week; have a score ≥5 (maximum score of 8) on the CAPS nightmare item (item B2, “recurrent distressing dreams of the event”); and be willing to continue maintenance psychotropics (stable dosage for at least the 4 weeks prior to randomization) during the trial.
Medical exclusion criteria included acute or unstable medical illness; systolic blood pressure <110 mmHg supine or orthostatic hypotension (systolic blood pressure decrease from supine >20 mmHg after 2 minutes standing or any decrease accompanied by dizziness). Women were excluded if pregnant, currently nursing, or unwilling to use reliable birth control. Psychiatric and behavioral exclusion criteria, based on assessment with the Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (
16), included psychotic disorders, cognitive disorders, substance abuse or dependence within the past 3 months, current cocaine or psychostimulant use, active suicidal or homicidal ideation, and depression requiring psychiatric hospitalization. Patients were also excluded if they were currently taking prazosin or any other alpha-1 adrenoreceptor antagonist or had a previous trial of prazosin for PTSD; if they had received prolonged exposure therapy, cognitive processing therapy, or eye movement desensitization and reprocessing therapy within 4 weeks before randomization; and if they had taken trazodone within 2 weeks of randomization (trazodone may increase the small risk of priapism associated with alpha-1 adrenoreceptor antagonists).
Assessments
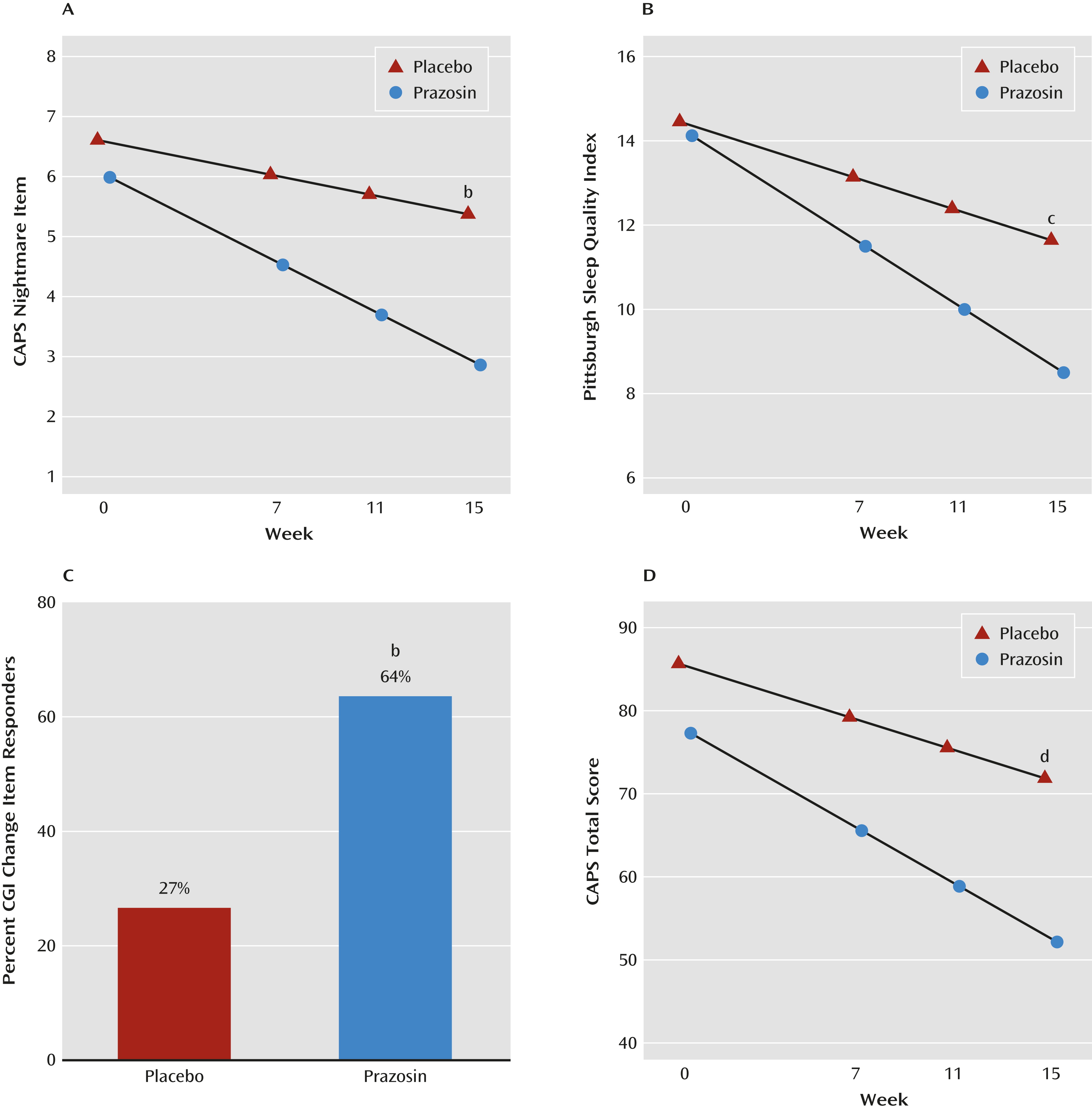
The three prospectively designated primary outcome measures were the CAPS nightmare item, the Pittsburgh Sleep Quality Index (
17), and the Clinical Global Impressions Scale (CGI) change item (
18) operationalized as treatment impact on self-reported ability to function in daily activities. Secondary outcome measures were the 17-item CAPS; the three CAPS symptom clusters (reexperiencing, avoidance, and hyperarousal); the Hamilton Depression Rating Scale (HAM-D) (
19); the Patient Health Questionnaire–9 (PHQ-9) (
20); and the Quality of Life Inventory (
21). Combat exposure was quantified with the Combat Experiences Scale (
1), which was designed for deployment in Iraq and Afghanistan. Blood pressure after 5 minutes supine and after 2 minutes standing, along with any reported adverse events, were recorded at all visits. Behavioral ratings were obtained at baseline and at weeks 7, 11, and 15.
Statistical analyses for the primary, secondary, and exploratory outcome analyses followed the intent-to-treat principle. Differences between treatment groups in 15-week change from baseline were assessed using linear mixed-effects models, which include all participants and allow for missing values (
22). These models included terms for gender, antidepressant use, week, treatment group, and, except in the case of the CGI change item, a week-by-treatment group interaction term; participants were treated as a random effect. Results for baseline and end of study are reported as adjusted means with the covariates gender and antidepressant use set to their average values. Results for differences between treatment groups in 15-week change from baseline are based on the week-by-treatment group interaction term.
Because the CGI change item inherently measures change from baseline, the linear mixed-effects model for this outcome measure did not include a week-by-treatment group interaction term and reported differences between treatment groups reflected the difference in CGI change item at week 15. Participants who dropped out before the week 7 visit did not have any CGI change item measures and were not included in the analyses of this measure.
As a secondary analysis, the 7-point CGI change item was collapsed into “responders,” defined as having markedly or moderately improved (scores of 1 or 2) and “nonresponders,” defined as minimally improved, no change, minimally worse, moderately worse, or markedly worse (scores of 3–7). The difference between treatment groups in the proportion of responders was assessed using a generalized linear-effects model with antidepressant use and gender as covariates.
An exploratory analysis addressed the possible effects of concurrent SSRI use (the predominant antidepressant class used) on change in the CAPS total score and the three primary outcome measures. For these analyses (except the CGI change item), the models included a week-by-treatment group-by SSRI use interaction term to determine whether the difference in change from baseline between treatment groups differed by SSRI use. The model for the CGI change item included a treatment group-by-SSRI use interaction term.
Adverse events were classified into 12 categories, as well as a category for any adverse event. The proportion of participants who experienced at least one adverse event in a category was compared between treatment groups using Fisher’s exact test. Confidence intervals for the differences between proportions were computed based on inverting the score statistic.
All analyses used R, version 2.15.1 (
23). Linear mixed-effects models were fitted using the lme function in the R package nlme (
24), and generalized linear mixed-effects models were fitted using the glmer function in the R package lme4 (
25).