Effect of Substance Dependence on Striatal Prediction Error
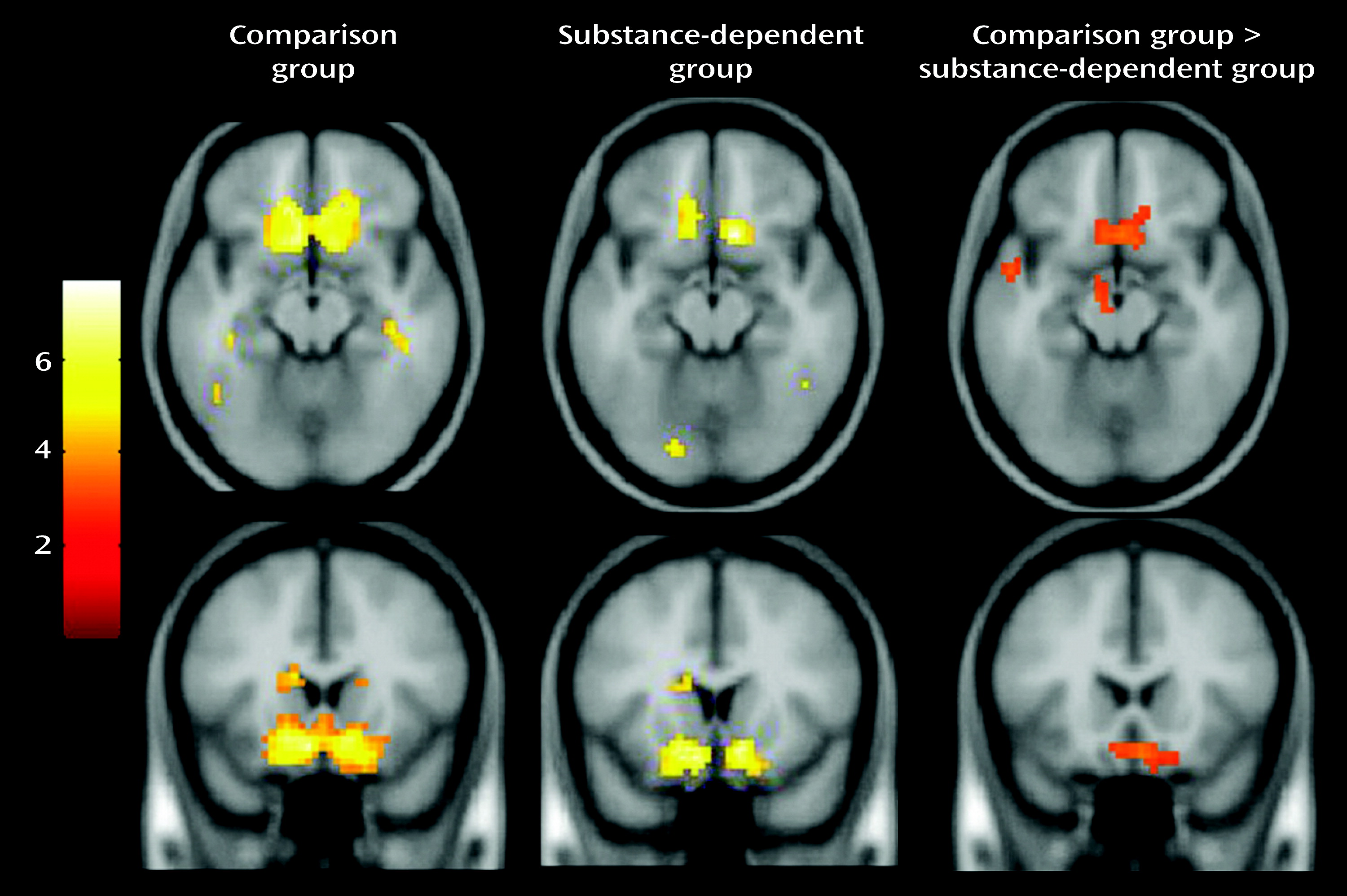
Relative to comparison subjects, substance-dependent individuals showed a significantly weaker relationship between prediction error and fMRI signal in the ventral striatum and medial orbitofrontal cortex. This finding is consistent with a large body of evidence of altered striatal function related to drug abuse. While it is firmly established that drugs act on the striatal dopaminergic reward circuitry, the development of addiction also likely involves non-reward processes involving learning and decision making (
5). Cue learning, for example, is driven by dopaminergic signals that track prediction error and is thought to be altered in addiction. Animals exposed to cocaine show abnormal striatal firing during odor discrimination learning in which rats learned that one odor predicted sucrose and another predicted quinine (
3). Cue-selective activity was recorded in dopaminergic striatal neurons. Control rats showed stronger cue-selective activity in the ventral striatum compared with cocaine-treated rats, which is consistent with our results. We found no group difference in activity in the dorsal striatum, which is somewhat inconsistent with other studies. Cocaine addicts, for example, show greater dorsal striatal dopamine release when viewing drug-related compared with neutral videos, suggesting that cocaine shifts the representation of learning signals from the ventral striatum, which involves reward and motivation, to the dorsal striatum, which involves habit learning (
31). Dorsal striatal activity is hypothesized to prime individuals to engage in habitual behaviors, such as procuring drugs (
6,
32). Our finding that prediction error tracking was represented in the dorsal striatum for both groups, but with no difference between them, may be related to the fact that our decision-making task is unlikely to induce the strong habit-learning that would be expected for drug cues.
Effect of Substance Dependence on Orbitofrontal Cortex Expectancies
Cue-selective firing during prediction learning is not unique to the striatum but is also found in prefrontal cortex neurons (
33,
34). Stalnaker et al. (
35) showed that cocaine-treated rats fail to develop cue-selective firing in orbitofrontal cortex neurons using the odor discrimination task described above. Furthermore, when the odor-outcome pairings were reversed, these neurons failed to demonstrate normal reversal of cue-selective firing in cocaine-treated compared with saline-treated rats, suggesting that drugs disrupt learning signals processed in the orbitofrontal cortex. Our finding of weaker tracking of prediction error in the medial orbitofrontal cortex in patients relative to comparison subjects is consistent with these studies. Drugs may disrupt outcome expectancies encoded in the orbitofrontal cortex and prevent the striatum from accurately updating prediction error (
34). This is consistent with reduced frontal-striatal connectivity reported in alcohol-dependent patients and suggests a disruption in the transfer of prediction error signals from the striatum to the orbitofrontal cortex (
9). Another hypothesis is that dopaminergic striatal activity serves as a gating signal to the prefrontal cortex and that drug exposure dampens the ability of natural rewards to open the gating so that the orbitofrontal cortex can influence decision making (
5).
Our finding that group differences in prediction error involved the medial more than the lateral orbitofrontal cortex confirms the critical role of the ventromedial prefrontal cortex in performance on the Iowa Gambling Task (
27). It also supports the notion that the medial and lateral orbitofrontal cortex play distinct roles in reward-related decision making. The medial orbitofrontal cortex has been more strongly associated with motivation, uncertainty, valuation, and effort. The lateral orbitofrontal cortex has been associated with processing aversive outcomes (
36) or suppressing unrewarded responses (
37). The lateral orbitofrontal cortex is thought to exert top-down control, planning how to achieve goals and passing the information to the medial orbitofrontal cortex, which evaluates the cost-benefit balance of those plans and estimates and monitors expected value (
38). In our study, fMRI signal in the orbitofrontal cortex correlated positively with response consistency and negatively with recency valence estimates in comparison subjects but not in patients, suggesting that the orbitofrontal cortex plays a role in computing expected valence and may be affected in drug users. These correlates were weak, however, and would need to be replicated.
Model-Based fMRI in Drug Users
Although this was not the first study to investigate neural correlates of prediction error in addiction, it is the first to report differences in individuals with stimulant dependence. Park et al. (
9) reported no difference in striatal prediction error in alcohol-dependent compared with healthy men performing a probabilistic learning task, while Chiu et al. (
8) found no difference in smokers compared with nonsmokers playing a stock market simulation task. Our study differs from these studies in several ways. First, the substance use problems in our population were more severe. Second, we used a non-commercially available z-shim MR pulse sequence that increases signal in the orbitofrontal region (
29). Third, the study tasks differed in cognitive demand. Finally, the use of internally generated (i.e., subjective) prediction error signals could be more sensitive than externally computed (i.e., objective) prediction error signals. For example, Stout et al. (
39) showed that a cognitive model of internally generated valence predicted behavior on the Iowa Gambling Task better than a linear model based on externally computed valence, consistent with our finding that the valence model described task behavior better than a conventional reinforcement learning model. We argue that brain activity should more closely track internally generated compared with externally computed expectancy.
Cognitive Processes Underlying Decision Making on the Modified Iowa Gambling Task
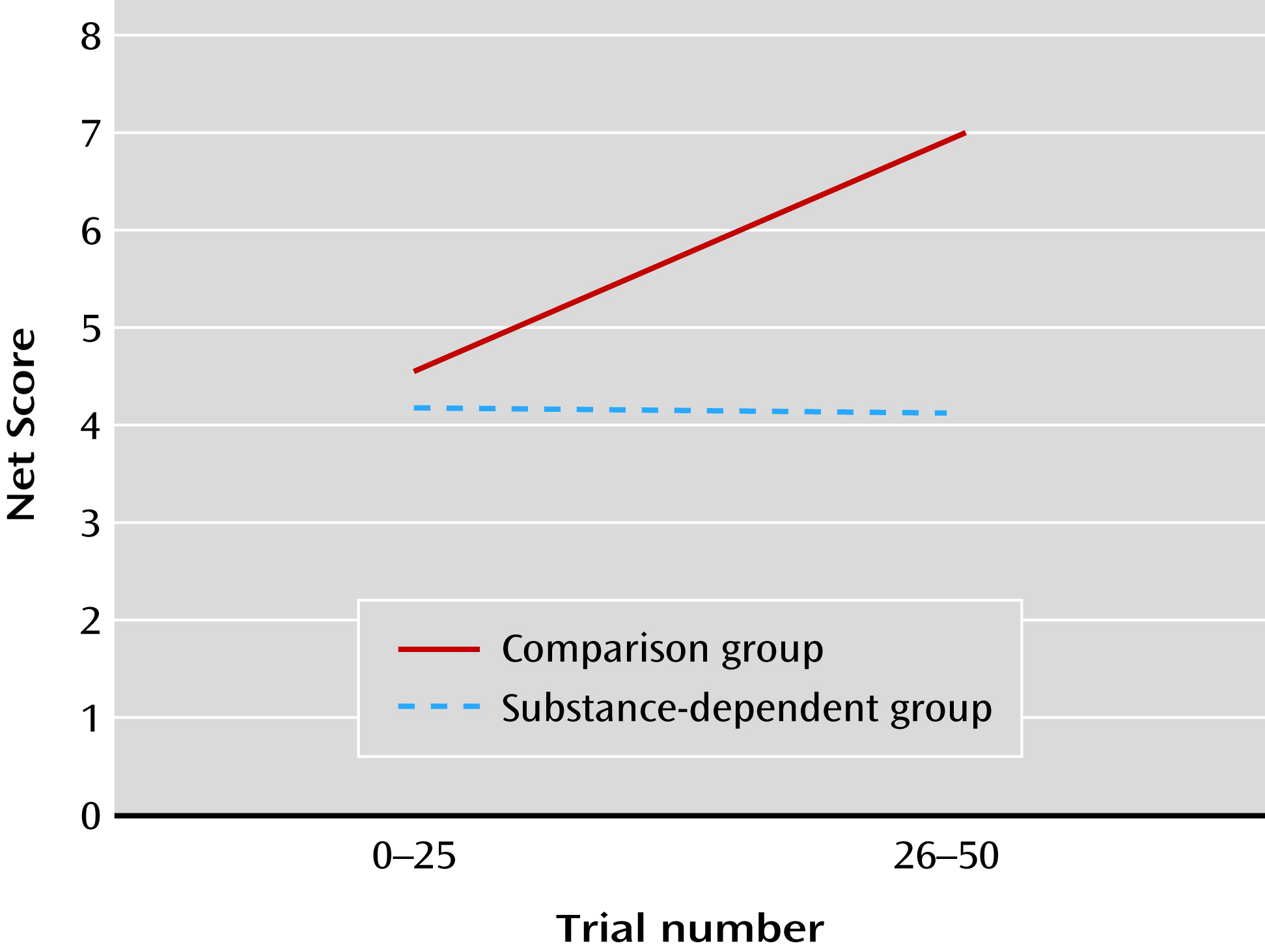
Relative to the comparison group, the patient group did not show an improvement in decision making over time, consistent with previous studies (
12,
40). Performance on the Iowa Gambling Task is influenced by many factors, including reward sensitivity, ability to forgo short-term in favor of long-term gain, impulsiveness, and cognitive flexibility. The expectancy valence model disentangles some of the processes (
14) by parameterizing sensitivity to gain and loss (risk), discounting of prior experiences (recency), and consistency between expectancies and choice (
28). Despite the modifications to the original Iowa Gambling Task (i.e., subjects were not free to select but rather a deck was selected for them), our results were consistent with previous model studies in drug users (
28,
41). The finding that substance users’ decisions were less consistent and less sensitive to loss than comparison subjects’ may reflect impulsiveness and “myopia for the future.”
Most neuroimaging studies of the Iowa Gambling Task model specific trial conditions (i.e., decision versus no decision or high versus low risk). Brain regions implicated in these models include the medial and lateral prefrontal cortex, the cingulate cortex, the striatum, and the insula (
16–
19). These models, however, fail to account for learning. To approximate this critical aspect of the task, Li et al. (
18) compared brain activity from the first and last blocks of the task and found that orbitofrontal cortex activity increased over time. In contrast, Lawrence et al. (
19) reported less activity in the orbitofrontal cortex and insula over time. One possibility for these inconsistencies is that individuals learn at different rates. Model-based fMRI accounts for variations in learning and has been applied to the Iowa Gambling Task (
18,
23). Christakou et al. (
23) found that the right ventromedial prefrontal cortex was sensitive to individuals’ negative expectancies. Although we did not separate positive from negative expectancies, our finding of ventral medial activity related to global expectancy is consistent with these results (
23). Li et al. (
18) also found that prediction error correlated with activity in the ventral striatum and the middle frontal gyri. Both of these previous studies, however, used externally derived prediction estimates. Our study extends model-based fMRI studies for the first time using internally derived prediction estimates in substance-dependent individuals.
Strengths of this study include the use of a computational model based on internally generated valence, the relatively large sample size of 32 patients, the use of an MR pulse sequence that increases orbitofrontal cortex signal compared with conventional MRI sequences, and a comparison group matched on IQ. Limitations include an inability to directly compare results to the Iowa Gambling Task, an inability to determine whether drug use preceded or antedated neural changes, the abuse of multiple drug classes in our sample, and the lack of control for medications. We cannot exclude the possibility that antisocial personality disorder could account for some results, as this diagnosis was common in both groups.
In summary, decision-making deficits in substance users compared with healthy subjects were related to inconsistent choices and reduced neural tracking of prediction error signals in the striatum and orbitofrontal cortex, suggesting that frontal-striatal pathways involved in learning may be a target for future treatment.