A Double-Blind Randomized Controlled Trial of Augmentation and Switch Strategies for Refractory Social Anxiety Disorder
Abstract
Objective
Method
Results
Conclusions
Method
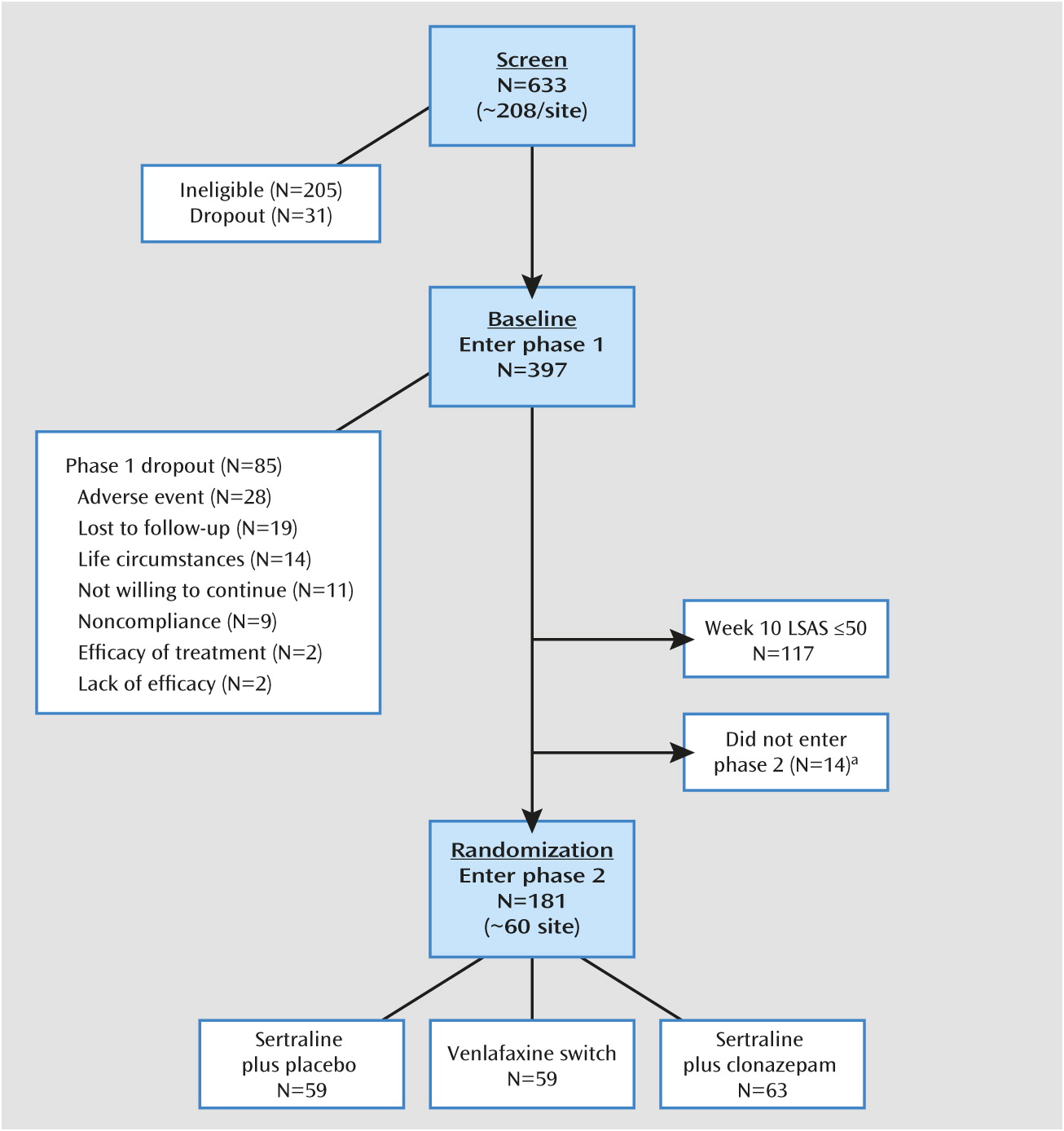
Participants
| Characteristic | Sertraline Plus Clonazepam (N=63) | Sertraline Plus Placebo (N=59) | Venlafaxine (N=59) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 34.8 | 12.3 | 35.3 | 14.3 | 33.7 | 12 |
| N | % | N | % | N | % | |
| Site | ||||||
| Massachusetts General Hospital | 21 | 33 | 19 | 32 | 19 | 32 |
| University of California, San Diego | 16 | 25 | 15 | 25 | 15 | 25 |
| McMaster University | 26 | 41 | 25 | 42 | 25 | 42 |
| Male | 38 | 60 | 39 | 66 | 38 | 64 |
| Female | 25 | 40 | 20 | 34 | 21 | 36 |
| Race | ||||||
| Asian | 8 | 13 | 10 | 17 | 3 | 5 |
| Black/African American | 4 | 6 | 1 | 2 | 3 | 5 |
| White | 50 | 79 | 44 | 75 | 48 | 81 |
| Other | 1 | 2 | 4 | 7 | 5 | 8 |
| Ethnicity | ||||||
| Hispanic | 3 | 5 | 5 | 8 | 3 | 5 |
| Non-Hispanic | 60 | 95 | 54 | 92 | 56 | 95 |
| Week 10 social anxiety disorder severity (Liebowitz Social Anxiety Scale) | ||||||
| Total score ≤70 | 28 | 40 | 27 | 46 | 26 | 44 |
| Total score >70 | 35 | 56 | 32 | 54 | 33 | 56 |
| Phase 1 instructions received | ||||||
| Exposure | 38 | 60 | 26 | 44 | 29 | 49 |
| Neutral | 25 | 40 | 33 | 56 | 30 | 51 |
| Study completion status | ||||||
| Discontinued early | 6 | 10 | 9 | 15 | 12 | 20 |
| Completed | 57 | 90 | 50 | 85 | 47 | 80 |
| History of psychotropic use | 28 | 44 | 31 | 53 | 25 | 42 |
| Comorbidity | ||||||
| Major depressive disorder | ||||||
| Current (past 2 weeks) | 11 | 17 | 15 | 25 | 12 | 42 |
| Lifetime | 33 | 52 | 28 | 47 | 30 | 51 |
| Other anxiety disorder | ||||||
| Current | 26 | 41 | 24 | 41 | 19 | 32 |
| Lifetime | 28 | 44 | 25 | 42 | 19 | 32 |
| Panic disorder | ||||||
| Current | 7 | 11 | 3 | 5 | 7 | 12 |
| Lifetime | 9 | 14 | 5 | 8 | 7 | 12 |
| Generalized anxiety disorder | ||||||
| Current (past 6 months) | 23 | 37 | 21 | 36 | 16 | 27 |
| Lifetime | 23 | 37 | 21 | 36 | 16 | 37 |
| OCD | ||||||
| Current (past 6 months) | 6 | 10 | 7 | 12 | 4 | 7 |
| Lifetime | 7 | 11 | 7 | 12 | 4 | 7 |
| PTSD | ||||||
| Current | 1 | 2 | 4 | 7 | 0 | 0 |
| Lifetime | 2 | 3 | 4 | 7 | 1 | 2 |
| Adult ADHD | ||||||
| Current (past 6 months) | 2 | 3 | 5 | 8 | 3 | 5 |
| Lifetime | 2 | 3 | 5 | 8 | 3 | 5 |
| Any lifetime eating disordera | 0 | 0 | 1 | 2 | 1 | 2 |
| Lifetime alcohol abuse or dependencea | 3 | 5 | 7 | 12 | 10 | 17 |
| Lifetime drug abuse or dependencea | 5 | 8 | 3 | 5 | 6 | 10 |
Study Design and Procedures
Measures
Clinician-rated instruments.
Self-report questionnaires.
Statistical Methods
Results
Overview
Remission
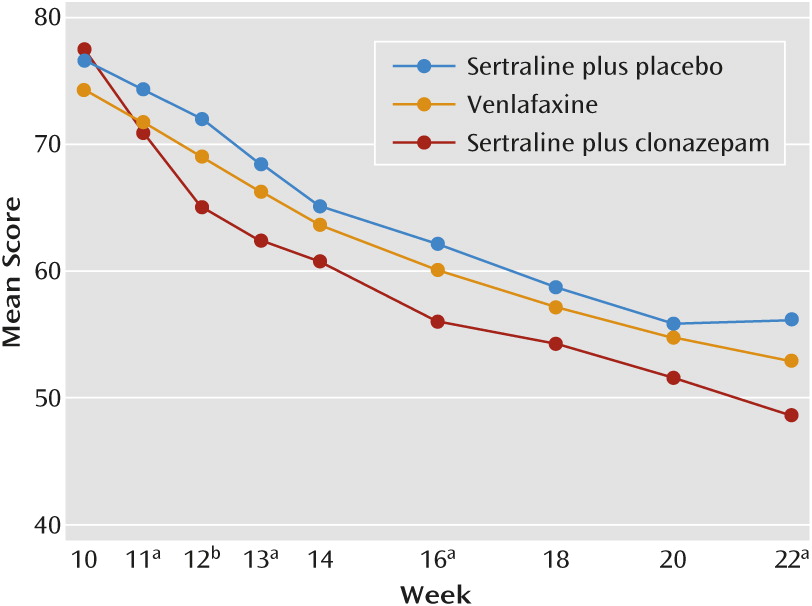
Change in LSAS Score
| Comparison Outcomea | Sertraline Plus Clonazepam (N=63) | Sertraline Plus Placebo (N=59) | Venlafaxine (N=59) | Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | β Estimate | SE | χ2 (df=1) | p | |
| Remission (LSAS≤30) | 17 | 27 | 10 | 17 | 11 | 19 | ||||
| Sertraline+clonazepam and sertraline+placebo | 0.62 | 0.47 | 1.74 | 0.19 | ||||||
| Venlafaxine and sertraline+placebo | 0.32 | 0.51 | 0.41 | 0.52 | ||||||
| Sertraline+clonazepam and venlafaxine | 0.30 | 0.47 | 0.40 | 0.53 | ||||||
| Response (LSAS≤50) | 35 | 56 | 21 | 36 | 27 | 46 | ||||
| Sertraline+clonazepam and sertraline+placebo | 0.93 | 0.42 | 4.91 | 0.029b | ||||||
| Venlafaxine and sertraline+placebo | 0.70 | 0.43 | 2.67 | 0.10 | ||||||
| Sertraline+clonazepam and venlafaxine | 0.23 | 0.41 | 0.30 | 0.58 | ||||||
| Mean | SD | Mean | SD | Mean | SD | β Estimate | SE | t (df=1) | p | |
| Change in LSAS Score | –26.5 | 24.4 | –16.5 | 23.0 | 17.6 | 19.1 | ||||
| From week 10 to last visit (intention to treat) | ||||||||||
| Sertraline+clonazepam and sertraline+placebo | –8.78 | 3.75 | –2.34 | 0.020b | ||||||
| Venlafaxine and sertraline+placebo | –2.14 | 3.81 | –0.56 | 0.57 | ||||||
| Sertraline+clonazepam and venlafaxine | –6.64 | 3.77 | –1.76 | 0.080 | ||||||
| From week 10 to week 22 (completers) | –28.5 | 24.2 | –19.6 | 22.4 | –21.7 | 19.0 | ||||
| Sertraline+clonazepam and sertraline+placebo | –9.16 | 4.16 | –2.20 | 0.029b | ||||||
| Venlafaxine and sertraline+placebo | –2.34 | 4.38 | –0.53 | 0.59 | ||||||
| Sertraline+clonazepam and venlafaxine | –6.82 | 4.24 | –1.61 | 0.11 | ||||||
Response
Dosing and Treatment Tolerability
Secondary Outcomes
| Measure and Comparisona | Sertraline Plus Clonazepam (N=63) | Sertraline Plus Placebo (N=59) | Venlafaxine (N=59) | Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | β Estimate | SE | t (df=1) | p | |
| CGI-I Score (R2=0.19) | ||||||||||
| Last visit | 2.2 | 1.1 | 2.5 | 1.2 | 2.3 | 1.2 | ||||
| Sertraline+clonazepam and sertraline+placebo | –0.24 | 0.19 | –1.22 | 0.23 | ||||||
| Venlafaxine and sertraline+placebo | –0.25 | 0.20 | 1.26 | 0.21 | ||||||
| Sertraline+clonazepam and venlafaxine | 0.013 | 0.07 | 0.07 | 0.95 | ||||||
| MADRS total score (R2=0.066) | ||||||||||
| Week 10 | 8.7 | 6.6 | 7.0 | 6.0 | 8.3 | 7.5 | ||||
| Last visit | 6.7 | 5.7 | 6.3 | 7.0 | 6.7 | 6.7 | ||||
| Change | –2.1 | 5.9 | –0.8 | 5.1 | –1.6 | 4.5 | ||||
| Sertraline+clonazepam and sertraline+placebo | –1.27 | 0.93 | –1.37 | 0.17 | ||||||
| Venlafaxine and sertraline+placebo | –0.88 | 0.95 | –0.91 | 0.37 | ||||||
| Sertraline+clonazepam and venlafaxine | –0.42 | 0.93 | –0.44 | 0.66 | ||||||
| SDS total score (R2=0.18) | ||||||||||
| Week 10 | 14.2 | 5.7 | 11.5 | 5.8 | 14.0 | 6.1 | ||||
| Last visit | 10.0 | 6.1 | 10.1 | 6.8 | 11.1 | 6.8 | ||||
| Change | –4.3 | 5.5 | –1.4 | 5.2 | –2.9 | 4.4 | ||||
| Sertraline+clonazepam and sertraline+placebo | –2.62 | 0.86 | –3.03 | 0.0028b | ||||||
| Venlafaxine and sertraline+placebo | –1.69 | 0.88 | –1.91 | 0.058 | ||||||
| Sertraline+clonazepam and venlafaxine | –0.93 | 0.88 | –1.07 | 0.29 | ||||||
| HAM-A total (R2=0.051) | ||||||||||
| Week 10 | 10.2 | 5.4 | 10.3 | 6.9 | 9.8 | 6.6 | ||||
| Last visit | 7.9 | 5.5 | 9.0 | 6.7 | 8.3 | 5.1 | ||||
| Change | –2.3 | 5.3 | –1.3 | 5.8 | –1.5 | 5.0 | ||||
| Sertraline+clonazepam and sertraline+placebo | –0.88 | 0.97 | –0.91 | 0.36 | ||||||
| Venlafaxine and sertraline+placebo | –0.19 | 0.98 | –0.19 | 0.85 | ||||||
| Sertraline+clonazepam and venlafaxine | –0.70 | 0.97 | –0.72 | 0.48 | ||||||
| Q-LES-Q total (R2=0.11) | ||||||||||
| Week 10 | 47.1 | 8.0 | 47.9 | 8.0 | 45.9 | 8.5 | ||||
| Last visit | 49.9 | 8.2 | 50.1 | 8.9 | 48.1 | 8.4 | ||||
| Change | 2.8 | 6.5 | 2.2 | 5.4 | 2.2 | 5.2 | ||||
| Sertraline+clonazepam and sertraline+placebo | 0.40 | 1.01 | 0.40 | 0.69 | ||||||
| Venlafaxine and sertraline+placebo | 0.16 | 1.02 | 0.15 | 0.88 | ||||||
| Sertraline+clonazepam and venlafaxine | 0.24 | 1.01 | 0.24 | 0.81 | ||||||
Discussion
Acknowledgments
Footnote
Supplementary Material
- View/Download
- 469.76 KB
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

