Smoking during pregnancy affects not only mothers but also their offspring and has been identified as a leading cause of preventable illness (
1). Adverse outcomes begin in utero, with greater pregnancy-related complications, low birth weight, and stillbirth (
2). As early as infancy, exposed offspring demonstrate problems in attention and inhibitory gating (
3), often culminating in disruptive behavior disorders later in childhood (
4). Finally, as offspring pass through adolescence, higher rates of substance use and antisocial traits are reported (
5,
6).
One disorder that has received little attention in the context of prenatal tobacco exposure is bipolar disorder, a complex psychiatric syndrome associated with high levels of occupational and social impairment that affects approximately 0.5%−1.5% of the population (
7). Although mood disturbances comprise its core symptoms, manifestations also include a number of the externalizing behavioral problems observed among offspring exposed to smoking during pregnancy, including conduct problems, aggression, impulsivity, and hyperactivity (
8). Teenagers and adults with bipolar disorder also exhibit disproportionately high rates of smoking and substance use (
9,
10). Given the common clinical features between bipolar disorder and other psychiatric outcomes among exposed offspring, we examined whether exposure to smoking during pregnancy might contribute to the risk for bipolar disorder. We tested this question in the Child Health and Development Study (CHDS), an ethnically, educationally, and occupationally diverse and largely representative birth cohort from Alameda County, California. Using a nested case-control design, we examined the relationship between smoking during pregnancy and lifetime offspring risk for bipolar disorder, while accounting for possible demographic and pregnancy-related confounders.
Method
Sample
The cohort members were derived from the CHDS. During 1959–1966, this study recruited virtually all pregnant women receiving obstetric care from the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC) in Alameda County. Live offspring (N=19,044) were automatically enrolled in KPNC upon birth. Comprehensive data were collected from maternal medical records, maternal interviews, and other sources. Approximately 30% of the population of the county was enrolled in KPNC. KPNC membership was largely representative of the population of the Bay Area of California at the time, based on ethnicity, education, and occupation, although there was some underrepresentation of the extremes of income (
11). This cohort has been studied extensively for early developmental suspected causes of schizophrenia (
12); however, testing the relationship between smoking and bipolar disorder in offspring was an a priori hypothesis.
Case Subjects
Individuals with potential DSM-IV bipolar disorder (including bipolar I, bipolar II, and bipolar not otherwise specified) were ascertained using a screening procedure from at least one of three sources: KPNC; the Alameda County Behavioral Health Care (ABHCS) database; and a mailing of the entire living CHDS birth cohort (mothers and children), as detailed below. The purpose of three methods of ascertainment was to obtain as complete a pool of case and comparison subjects as possible. Individuals who were enrolled in KPNC by the first date of treatment were ascertained from this source. Individuals who lost KPNC or other health insurance and were still living in Alameda County would have been treated by ABHCS if they sought treatment. Individuals who were not ascertained by these two approaches were identified by a mailed survey that included questions on mental health treatment and was sent out to all mothers and cohort members in the CHDS and followed by a diagnostic screening interview.
KPNC.
Individuals with potential bipolar disorder were identified by screening the inpatient and outpatient databases of KPNC. Computerized record linkages between CHDS and KPNC identifiers were conducted on these databases. The inpatient database included all psychiatric hospitalizations of KPNC members, whether in KPNC or as referrals to non-KPNC hospitals, and covered the period from 1981 to 2010. The maximum duration of follow-up by this source was 29 years. Individuals from the KPNC inpatient database screened positive for bipolar disorder based on registry diagnoses of ICD-9 codes 295–298. The outpatient database had a limited introduction in 1981 and was subsequently extended to include all outpatient contacts for psychiatric care. Potential case subjects from the outpatient registry were considered positively screened if they were assigned ICD-9 diagnoses of 295–298 without unipolar major depressive disorder (296.3). Screening from the KPNC outpatient pharmacy database, beginning in 1992, complemented the case ascertainment. Individuals from this source screened positive if they received lithium, carbamazepine, or valproic acid. Before contacting individuals who were enrolled in KPNC, the treating psychiatrist was contacted, informed about the study, and asked to approve contact with the potential case subject to seek his or her consent to participate.
Individuals identified by any of these methods were invited to participate in the study by letters mailed to their most recent address. Those who did not refuse contact were contacted by a staff member, who arranged an appointment for a diagnostic interview. Potential case subjects no longer living at the most recent listed address were located using a variety of sources, including the Department of Motor Vehicles (DMV), telephone directories, the individuals’ parents (or other relatives), mortality records, reverse directories, jail searches, and visits to previous addresses.
ABHCS.
Potential case subjects were also determined by electronic record linkage between the CHDS and ABHCS identifiers; the ABHCS database covered treatment from 1993 to 2009. These individuals screened positive based on ICD-9 inpatient diagnoses of 295–298; for outpatients, these same diagnoses were used, excluding major depressive disorder. Procedures for recruitment and location of these potential case subjects were similar to those described above for ascertainment by KPNC.
Mailed questionnaire and follow-up.
The third method of ascertainment involved a mailed questionnaire on mental and physical health to all mothers (N=6,971) and cohort members (N=13,009) in the CHDS cohort with known addresses (excluding families where potential case subjects had already been identified from KPNC or ABHCS); a request for contact information was also included. This protocol was conducted from 2009 to 2011. Respondents who reported mental health problems in an eligible cohort member (including in the respondent him- or herself) were contacted by a trained KPNC study interviewer. In order to identify offspring in the birth cohort with potential bipolar disorder, the interviewer administered the Family Interview for Genetic Studies (
https://www.nimhgenetics.org/interviews/figs/). Any cohort member with at least one symptom of bipolar disorder or psychosis was considered to have screened positive and was invited for a diagnostic interview. The total number of case subjects ascertained from these three sources was 448.
Diagnostic Protocol
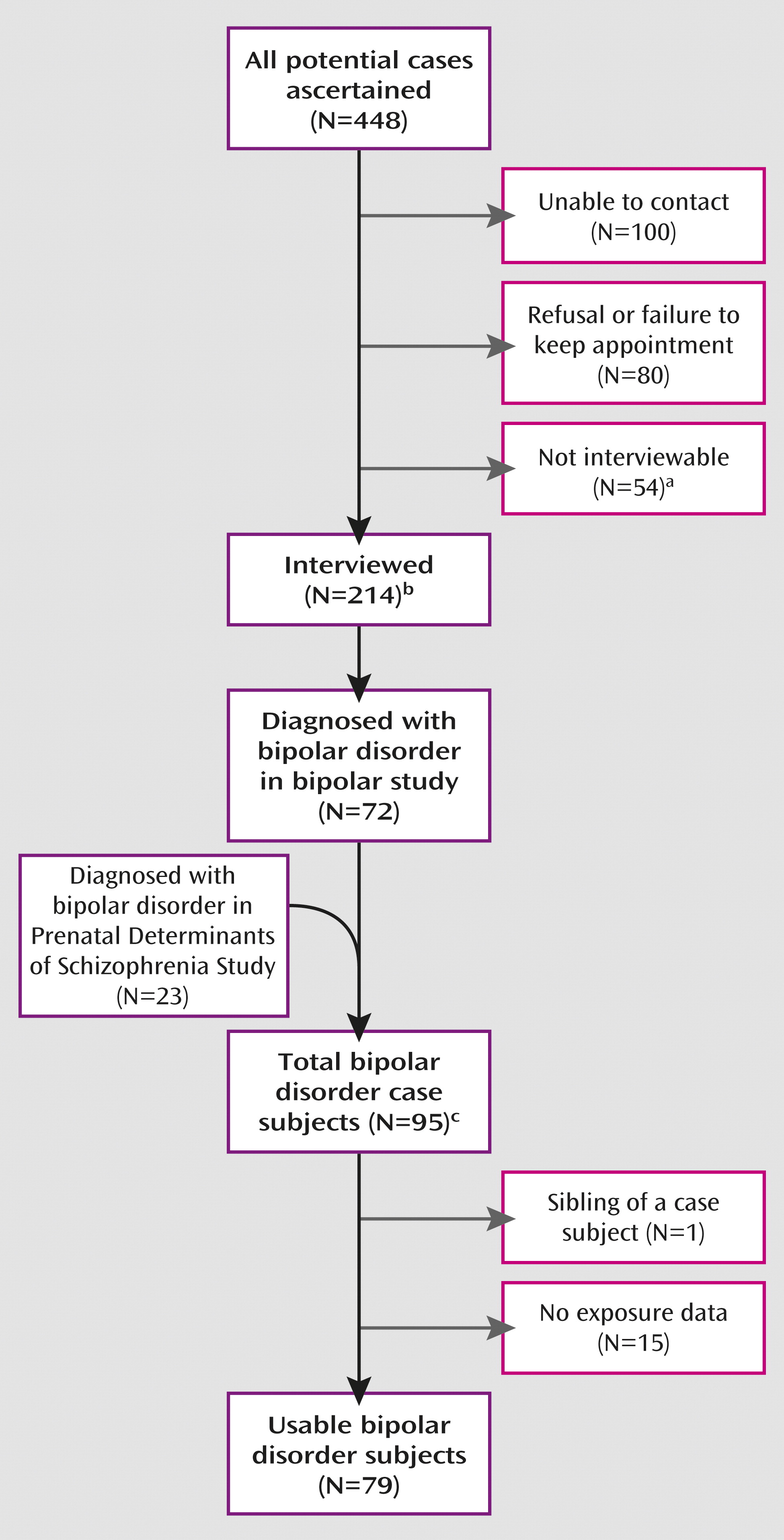
We targeted all potential case subjects identified from the above procedures to schedule a detailed diagnostic interview with the Structured Clinical Interview for DSM-IV (SCID). A total of 214 individuals were interviewed (
Figure 1). DSM-IV-TR diagnoses, including diagnostic qualifiers representing subtypes of bipolar disorder, were systematically assigned by consensus of three experienced clinicians (research psychiatrists and a Ph.D. psychologist), based on a diagnostic conference call with the interviewers and a review of the SCID information, including a written narrative summarizing the interview and any available information from medical records. Information was supplemented with inpatient or outpatient psychiatric records for potential case subjects for whom a definitive diagnosis could not be made from the SCID. This protocol resulted in 72 bipolar disorder case subjects.
Additional bipolar case subjects ascertained by KPNC in an earlier study, Prenatal Determinants of Schizophrenia I (PDS-I), were included. Although the PDS-I study targeted schizophrenia and related schizophrenia spectrum case subjects, bipolar disorder case subjects were also diagnosed from interview in that study because the assessment instrument included bipolar disorder symptoms. The protocol for the PDS-I study (
12) also included electronic database linkages with the KPNC inpatient, outpatient, and pharmacy registries, from 1981 to 1998. The only differences in ascertainment and screening between the PDS-I and KPNC protocols were that the PDS-I study did not include data on treatment with antimanic medications but included a psychiatrist's review of inpatient and outpatient records for psychotic symptoms. The 183 potential case subjects identified from this source were interviewed using the Diagnostic Interview for Genetic Studies (
13); 23 individuals with bipolar disorder were diagnosed. Taken together with the patients diagnosed in the present study, a total of 95 bipolar disorder case subjects were diagnosed following ascertainment from all sources.
Matched Comparison Subjects
The first step in selecting comparison subjects was to exclude members of the CHDS cohort who screened positive for potential psychoses or bipolar disorder (N=413), that is, those who did not meet any of the screening criteria described above. In order to maximize statistical power, we selected comparison subjects from the CHDS cohort who were matched up to 8:1 to bipolar disorder cases on several criteria. First, to ensure that each case and his or her corresponding comparison subject were followed for equal time from birth to first treatment for the case subject, comparison subjects were matched to case subjects on membership in KPNC (for case subjects ascertained through KPNC) or on residence in Alameda County (for case subjects ascertained through ABHCS or by the CHDS mailing survey and protocol) in the year the individual was first treated. Selection by this protocol allowed us to define representative samples for the respective source populations of case subjects (i.e., individuals who would have been diagnosed as case subjects if they developed bipolar disorder). For KPNC, membership at the time of diagnosis was used for comparison matching because members would have been documented in KPNC databases if they sought care for bipolar disorder. For case subjects treated by ABHCS, the source population at the time of diagnosis was determined with DMV records indicating residence in Alameda County, because these individuals represented the population at risk for treatment in ABHCS if they were diagnosed with bipolar disorder. For case subjects ascertained by the mailed survey to all living CHDS mothers and cohort members, the source population was also obtained by DMV records indicating residence in Alameda County because the vast majority of the individuals who received the mailing were residents of Alameda County. Potential comparison subjects matched to case subjects from ABHCS or the cohort mailing protocol and who belonged to KPNC at the time of determination were excluded from the comparison pool for those case subjects. Comparison subjects were matched to case subjects on date of birth (±30 days), sex, membership in the cohort at the time of illness onset, and availability of maternal archived sera (for serologic studies).
The comparison selection procedure consisted of first enumerating for every case the individuals who met these matching criteria. Those case subjects assigned to a matched set were removed from the potential comparison pool for subsequent case subjects until all matched sets were completed and a maximum of an 8:1 ratio of comparison to case subjects was achieved. Finally, we corrected the matched sample to include only one sibling per family because siblings represented nonindependent observations. This protocol yielded 754 matched comparison subjects; the corresponding case subjects and matched comparison subjects were termed a “matched set.”
Analytic Sample
The numbers of individuals ascertained, targeted for interview, interviewed, and diagnosed with bipolar disorder (including each subtype) are detailed in
Figure 1. All participants provided written informed consent for human investigation. The study protocol was approved by the institutional review boards of the New York State Psychiatric Institute and KPNC.
Derivation and Classification of Maternal Smoking Data
Data on maternal smoking were obtained by a comprehensive interview administered during pregnancy in CHDS. Exposure was classified as maternal smoking at any time during the current pregnancy; lack of exposure was defined as no maternal smoking at any time during pregnancy.
Covariates
Data on maternal age, education, race, and alcohol use were obtained from a comprehensive interview administered during pregnancy in the CHDS birth cohort. Data on delivery, birth weight, gestational age, sex, year of birth, maternal psychiatric history, and maternal medication use were systematically collected by CHDS staff from medical records, which were available for virtually all cohort members. All maternal psychiatric diagnoses were made by a physician and extracted from the records using the ICD coding.
Statistical Analysis
The individual associations summarized in
Tables 1 and
2 were tested using chi-square tests for dichotomous variables and t tests for continuous variables. Only variables that were significantly associated with prenatal smoking or bipolar disorder in offspring were included in subsequent models. Associations between maternal smoking during pregnancy and bipolar disorder in offspring were formally tested using conditional logistic regression models for matched sets. We first adjusted for each potential confounder individually to test its effect on the association between prenatal smoking and bipolar disorder. Variables whose inclusion changed the effect size of the association between prenatal smoking and bipolar disorder in offspring by more than 10% were retained in the final multiple regression model. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Mothers who smoked during pregnancy, as compared with those who did not, were more likely to be Caucasian and to use alcohol during pregnancy (
Table 1). They also had marginally higher rates of psychopathology. Severe psychopathology (schizophrenia or other psychotic disorders or depression) did not vary across the groups. The offspring of mothers who smoked during pregnancy had significantly lower birth weight than those whose mothers did not. Rates of bipolar disorder among the offspring did not vary by any maternal or offspring characteristics (
Table 2).
Offspring exposed to smoking during pregnancy had a nearly twofold greater risk for bipolar disorder (
Table 3, model A). This association remained essentially unchanged after adjusting for maternal race, alcohol use, psychiatric history, or offspring birth weight (
Table 3, model B). The quantity of maternal smoking also did not vary by bipolar disorder status in the offspring, with 54.2% of mothers of exposed case subjects and 60.5% of mothers of exposed comparison subjects smoking half a pack or more per day (χ
2=0.69, p=0.44). None of the above variables changed the primary associations for prenatal exposure by more than 10%. Inclusion of all of the above covariates into the analysis revealed that smoking during pregnancy was the strongest and only significant predictor of bipolar disorder in offspring (
Table 3, model C).
Finally, we stratified the analysis based on whether or not the bipolar disorder diagnosis had associated psychotic features. Among offspring without psychotic features (N=46) and matched comparison subjects, maternal smoking was associated with a more than twofold greater risk for the disorder in the full model (odds ratio=2.28, 95% confidence interval [95% CI]=1.20–4.31, p=0.026). Among offspring with psychotic features (N=33) and matched comparison subjects, maternal smoking was not significantly associated with bipolar disorder (odds ratio=1.75, 95% CI=0.79–3.87, p=0.169).
Discussion
Smoking during pregnancy was associated with an approximately twofold greater risk for bipolar disorder in offspring after accounting for all covariates tested. A number of studies, including at the population level, have documented adverse outcomes ranging from the prenatal period to early adulthood among offspring of mothers who smoke chronically during pregnancy (
5,
14). A recent Finnish birth cohort study, for example, found that exposed offspring had more emotional and behavioral problems in childhood, higher rates of psychotropic medication use, and more drug and alcohol use disorders (
5,
15). Although bipolar disorder was not specifically investigated, higher rates of mood disorders were observed.
Much of the psychopathology associated with prenatal tobacco exposure clusters around the externalizing spectrum, which includes attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder, conduct disorder, and substance use disorders (
6,
14,
16). Although not diagnostically classified along the externalizing spectrum, bipolar disorder shares a number of clinical characteristics with these disorders, including inattention, irritability, loss of self control, and proclivity to drug and alcohol use (
17,
18). Psychomotor agitation in mania may also be confused for the heightened motor activity common in the hyperactivity subtype of ADHD (
18). Perceptions of invincibility, antisociality, and delinquency are observed in bipolar disorder, conduct disorder, and antisocial personality disorder (
17). Among children with ADHD, comorbid conduct disorder may increase the likelihood of subsequent bipolar disorder (
19). Although the mechanisms are not fully understood, bipolar disorder may share some genetic risks with other externalizing disorders, as studies report familial aggregation between bipolar disorder and both ADHD (
18,
20) and conduct disorder (
21). Hence, although the small sample precludes formal conclusions, our observations in the bipolar disorder case subjects without psychotic features are consistent with the overall literature showing that outcomes of prenatal tobacco exposure are related to externalizing rather than psychosis-related disorders (
22).
The range of externalizing outcomes observed among offspring prenatally exposed to maternal smoking may reflect not only the pharmacological heterogeneity of tobacco (which contains more than 4,000 active ingredients, many of which, including nicotine, readily cross the placental barrier [
23]), but also the complexity of the nicotinic receptors themselves. The nicotinic acetylcholine receptor family comprises a number of receptors, including pentamer combinations of individually expressed subunits (
24). Nicotinic acetylcholine receptors densely innervate forebrain structures and are located presynaptically on dopaminergic and noradrenergic neurons, where they modulate synaptic plasticity and maintain an adequate signal-to-noise ratio in the developing cortex (
25). Nicotine, via presynaptic stimulation, can directly interact with the circuitry associated with mood regulation and executive control (
26). Chronic exposure can further phosphorylate subunits leading to permanent changes in receptor sensitivity. One receptor subtype particularly relevant to the phenomenology of bipolar disorder is the α7 nicotinic acetylcholine receptor, a homopentamer of five α7 subunits whose expression peaks in utero (
24) and is involved in regulating inhibitory interneuronal activity via GABA release, functioning of the hypothalamic-pituitary-adrenal (HPA) axis, and sensory gating, a semiautomated subcortical process that allows useful information to be extracted from sensory input (
27). Prenatal exposure can disrupt sensory gating, leading to impairments in cognitive and attention-related processes that manifest starting in infancy (
3).
CHRNA7 gene mutations have been linked to schizophrenia, bipolar disorder, and bipolar-type schizoaffective disorder (
28), and nicotinic agonists can induce manic episodes in persons with these disorders (
29).
Nicotine exposure may also restrict placental blood flow, placing the fetus at risk for malnutrition and low birth weight (
30), suspected causes in the development of bipolar disorder (
22). Thus, the impact of nicotine exposure may operate via multiple coexisting pathways. Finally, nicotine is one of thousands of potentially toxic compounds in tobacco (
31); others (e.g., carbon monoxide and harmans) also have known insidious effects and likely contribute to the adverse outcomes observed in the offspring.
The study has several strengths. First, maternal smoking was determined prospectively, during pregnancy, and thus the finding cannot be a result of retrospective bias in reporting of the exposure. Second, we used a well-characterized and population-based birth cohort. Third, case subjects were extensively followed up throughout adulthood using a combination of procedures aimed at maximizing recruitment. Fourth, diagnoses of bipolar disorder were made using direct, structured, research-based interviews followed by a systematic diagnostic consensus procedure.
A number of limitations should also be recognized. First, we cannot completely disaggregate effects of in utero from postnatal exposure, as mothers who smoke during pregnancy are also more likely to smoke after birth or to live with partners who smoke. Second, it is possible that some mothers underreported smoking. However, unless misclassification of smoking was also related to the likelihood that the offspring has bipolar disorder, this purported limitation would decrease the likelihood of detecting a difference. Generally, correlations between biological and self-reported measures of smoking among pregnant women are high (
32). Third, causal interpretations are unwarranted, as other unmeasured maternal factors (e.g., genetic variation, personality traits, HPA-axis activity, diet, life events, and lifestyle during or after pregnancy, including postnatal smoking) as well as paternal psychiatric history may have contributed to the relationships between maternal smoking and bipolar disorder in the offspring. Fourth, although smoking was reported prospectively, the present analysis relies on an overall measure of smoking provided at the time of the maternal interview, which was given at enrollment into the study, with the vast majority enrolling during the first and second trimesters. Fifth, influential matched sets could be an issue given the modest sample size; however, regression diagnostics could not be performed because of limited resources. Finally, because maternal psychiatric history was obtained by medical records rather than direct interview, it is possible that some psychiatric diagnoses were missed. However, it is unlikely that these would systematically vary by smoking history.
This is the first study to our knowledge to document associations between prenatal tobacco exposure and bipolar disorder. Given that smoking during pregnancy is potentially preventable by a number of measures, including antismoking campaigns and greater attention to smoking behaviors during obstetric visits, the findings could have clinical importance. However, if maternal smoking is simply serving as a proxy for deeper behavioral or genetic disturbances related to addictive behaviors that influence bipolar disorder, cessation per se may not have preventive effects.
Although smoke exposure cannot be randomized, future research should employ more semi-experimental approaches that will help both to establish specificity and to disentangle true teratogenic effects from other familial confounders. Because women who smoke will inevitably differ from those who do not on a variety of behavioral and possibly genetic measures that may be independently transmitted from mother to child, addressing such confounding effects remains a key issue in studies of prenatal exposures (
33–
35). Indeed, if smoking becomes less desirable, the proportion of smokers with psychiatric disorders is expected to increase (
36). Consequently, prenatal exposure effects may be increasingly difficult to dissociate from other maternal factors that are related to psychiatric disorders.
Ultimately, given that bipolar disorder is a complex disorder, prenatal exposure studies will require interpretation within the larger context of genetic and environmental risks on the developmental trajectory, recognizing that these risks may not be independent of one other.