Deficits in working memory are a core feature in patients with schizophrenia (
1). They are a strong predictor of functional outcomes (
2), and they are not improved by currently available pharmacologic agents. Abnormal cholinergic neurotransmission has been hypothesized to be a mechanism underlying working memory dysfunction in patients with schizophrenia (
3,
4). Notwithstanding the role of the dopaminergic system (especially through D
1 receptors) and the nicotinic system, muscarinic neurotransmission supports working memory through several mechanisms: enhanced discrimination of signal from noise, enhancement of dopaminergic neurotransmission, and dilation of cerebral blood vessels (see reference
5 for a recent review). However, to date, studies aimed at improving cholinergic neurotransmission have failed to improve cognition in patients with schizophrenia (
6,
7). The large variability in the cholinergic status of participants in these studies may have been problematic. Anticholinergic burden has been shown to be associated with cognitive impairment (
8), including working memory deficits (
9), in patients with schizophrenia. It has also been shown to prevent these patients from benefiting from procognitive interventions (
8).
This variability in cholinergic status could explain the mixed findings in the literature on the effects of clozapine on cognition or working memory performance in patients with schizophrenia. Studies assessing clozapine’s impact on cognition have shown mixed results, ranging from deleterious (
10–
12) to neutral (
13,
14) to beneficial effects (
15,
16). The differing actions of clozapine and its major active metabolite,
N-desmethylclozapine (NDMC), on the cholinergic system have been proposed as the explanation for these varying effects on cognition. Clozapine is an antagonist at muscarinic M
1, M
3, and M
5 receptors (
17,
18); thus, it is expected to have a detrimental effect on cognition, especially frontal working memory. In contrast, NDMC is a potent partial agonist at these sites and is expected to enhance working memory performance (
19,
20) (see
Table 1 for clozapine and NDMC affinities to various receptors). Notably, plasma concentrations of NDMC can vary from 20% to 150% of clozapine concentrations (
21). Thus, the cholinergic tone of individual patients and consequently their cognitive function should be more affected by the ratio of clozapine to NDMC than by either concentration alone.
Two published retrospective secondary data analyses support this hypothesis. One analysis (
22) assessed the relationship between executive function and exposure to clozapine and NDMC in patients with schizophrenia who had participated in two clinical trials. The investigators found that a low clozapine/NDMC ratio was associated with improvement in attention and working memory performance, and to a lesser degree executive function, while each concentration alone was not. In the other analysis (
23), the clozapine/NDMC ratio was more strongly associated with cognitive impairment, as assessed by a clinical measure of global cognition, than was either concentration alone. Thus, based on these results and on the role of muscarinic neurotransmission in working memory, we conducted a hypothesis-driven study designed to assess whether clozapine/NDMC ratio is a stronger determinant of cognition than clozapine and NDMC concentrations alone. We hypothesized that clozapine/NDMC ratio would predict working memory performance after controlling for age, gender, education, and symptom severity. We then tested the hypothesis of whether clozapine or NDMC concentration alone predicts working memory performance.
To assess the relationships between clozapine, NDMC, and the cholinergic burden, we explored the relationships between serum anticholinergic activity (SAA), clozapine concentration, NDMC concentration, and cognition.
Method
Participants
This cross-sectional study was conducted between February 2010 and December 2013 at the Centre for Addiction and Mental Health, a large academic psychiatric hospital that provides care to an urban catchment area and serves as a referral center for a large suburban and rural population in the Greater Toronto Area. Participants met DSM-IV-TR criteria for schizophrenia or schizoaffective disorder, were at least 18 years of age, and were clinically stable on clozapine monotherapy, taken once a day at bedtime. Clinical stability was operationalized as both not having been hospitalized within the past 3 months and having had no change in clozapine dosage within the past 4 weeks. The study was approved by the Centre for Addiction and Mental Health Research Ethics Board, and all participants provided written informed consent.
Assessments
On the morning that participants underwent their routine blood draw for white blood cell and absolute neutrophil count, their clinical and cognitive states were assessed and we collected a blood sample to measure clozapine and NDMC concentration and SAA.
Clinical and Cognitive Assessments
The Mini International Neuropsychiatric Interview (
24) was used to confirm diagnosis. Clinical symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS) (
25). Cognition was assessed on the day the blood was drawn with the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (
26). The MCCB includes 10 tests that assess seven cognitive domains: 1) processing speed (the symbol coding task from the Brief Assessment of Cognition in Schizophrenia, the category fluency test [animal naming], and the Trail Making Test, part A); 2) attention/vigilance (the Continuous Performance Test, Identical Pairs version); 3) working memory (the letter-number span test and the spatial span subtest of the WMS-III); 4) verbal learning (the Hopkins Verbal Learning Test–Revised); 5) visual learning (the Brief Visuospatial Memory Test–Revised); 6) reasoning and problem solving (the mazes test from the Neuropsychological Assessment Battery); and 7) social cognition (the managing emotions test from the Mayer-Salovey-Caruso Emotional Intelligence Test). Each domain T-score was used individually as an outcome measure.
Clozapine and NDMC Concentrations
Concentrations of clozapine and NDMC were determined from heparinized plasma by high-performance liquid chromatography with isocratic elution and ultraviolet detection at 245 nm (Waters instrumentation) using a procedure developed in-house as an adaptation from established methods (
27). The limit of quantitation was 100 nmol/L for both clozapine and NDMC, with a coefficient of variation for both of 8%–10%.
Serum Anticholinergic Activity (SAA)
SAA was determined using established procedures (
28). The limit of detection for SAA in this study was 0.25 pmol/mL with a standard linear curve (r=0.99) from 0.50 to 25.00 pmol/mL and a coefficient of variation <12%.
Statistical Analysis
Our primary analyses assessed the relationship between working memory performance and demographic, clinical, and pharmacological variables. We first computed a series of Spearman correlations between working memory performance (T-scores) on the one hand, and age, gender, education, PANSS score, clozapine concentration, NDMC concentration, and clozapine/NDMC ratio on the other. We then conducted an initial standard multiple regression analysis in which working memory performance was the dependent variable and age, gender, education, PANSS score, and clozapine/NDMC ratio were entered simultaneously as the independent variables. Finally, we conducted two further multiple regression analyses, replacing clozapine/NDMC ratio with clozapine concentration in one and NDMC concentration in the other.
Our first set of secondary analyses assesses whether the relationship between clozapine/NDMC ratio and working memory performance was driven by only one of the two working memory tests (letter-number span test and spatial span test) that contribute to the working memory domain in MCCB. Thus, we performed a series of analyses similar to those described above, except that working memory was replaced by each of the two tests separately.
Our second set of secondary analyses assessed the relationship between performance on the other six cognitive domains and demographic, clinical, and pharmacological variables. In these analyses, multivariate general linear models were applied. The dependent variables were speed of processing, attention/vigilance, verbal learning, visual learning, reasoning and problem solving, and social cognition. The covariates were age, gender, education, PANSS score, and clozapine concentration, NDMC concentration, or clozapine/NDMC ratio.
Our third set of secondary analyses explored whether the effects of clozapine and NDMC concentrations on cognition were related to cholinergic burden. First, we computed Spearman correlations between SAA on the one hand and clozapine and NDMC concentrations on the other, and between clozapine and NDMC concentrations. Second, we conducted a standard multiple regression analysis in which SAA was the dependent variable and age, gender, clozapine concentration, and NDMC concentration were entered simultaneously as the independent variables. Third, we conducted regression analyses similar to those with working memory performance and clozapine/NDMC ratio but replacing clozapine/NDMC ratio with SAA.
We used SPSS, version 15.0 for Windows (SPSS, Inc., Chicago), for all analyses.
Results
The demographic, clinical, cognitive, and pharmacological characteristics of the 30 participants who completed the study are summarized in
Table 2.
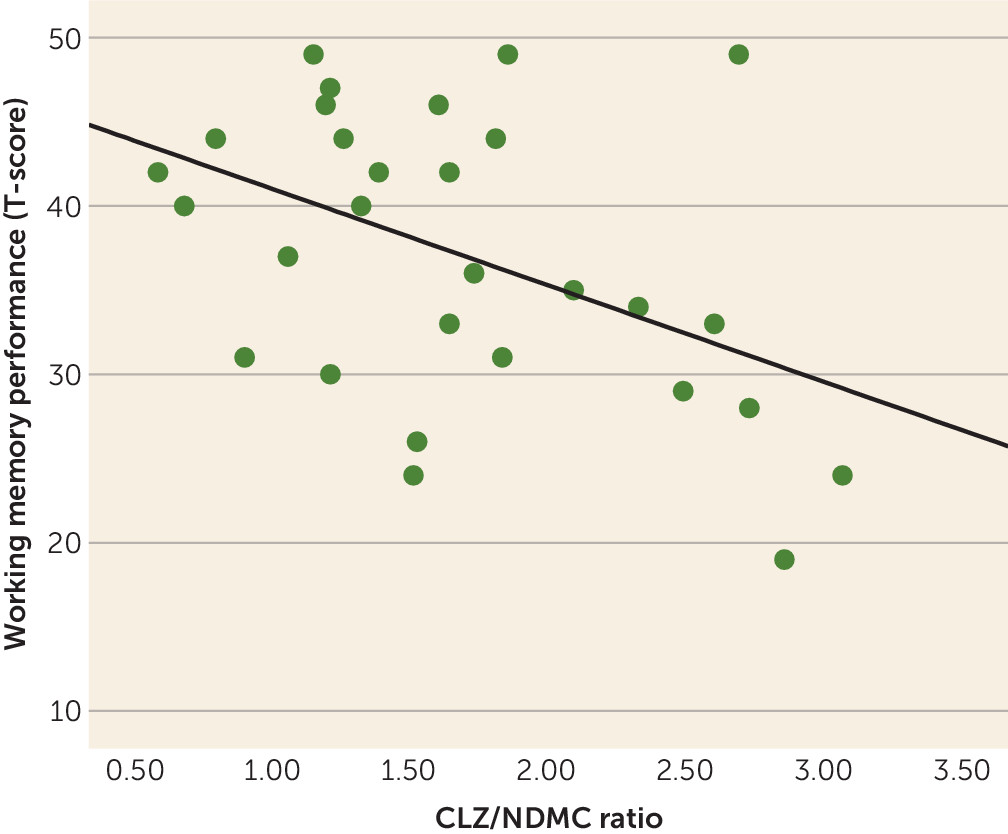
Working memory performance was negatively correlated with clozapine/NDMC ratio (
Figure 1) but was not correlated with age, gender, education, PANSS score, clozapine concentration, or NDMC concentration (
Table 3).
As shown in
Table 4, the results of the first multiple regression showed that working memory performance was associated with clozapine/NDMC ratio but not with age, gender, education, or PANSS score. In contrast, the results of the two multiple regressions with clozapine or NDMC concentration showed that working memory performance was not associated with either alone.
The multiple regressions with each of the working memory tests showed that there could be a trend toward significant associations between clozapine/NDMC ratio and verbal working memory performance (as assessed by the letter-number span test) or visual working memory performance (as assessed by the spatial span test), separately (letter-number span test: B=−4.68, SE B=2.49, β=−0.37, p=0.072; spatial span test: B=−4.12, SE B=2.20, β=−0.35, p=0.074). Also, they were not associated with clozapine concentration.
The multivariate general regression models assessing the relationships with the other six cognitive domains revealed no significant effects of clozapine concentration, NDMC concentration, clozapine/NDMC ratio, or SAA on performance in any of these six cognitive domains.
As expected, SAA was highly correlated with clozapine concentration (Spearman’s rho [rs]=0.83, p<0.001) and NDMC concentration (rs=0.66, p<0.001). Clozapine and NDMC concentrations were also highly correlated (rs=0.73, p<0.001). The multiple regression revealed an association of SAA with clozapine concentration (B=0.006, SE B=0.001, β=0.71, p<0.001) but not with NDMC concentration. The results of the multiple regression that included working memory performance as the dependent variable and SAA as independent variable revealed that SAA was not significantly associated with working memory performance.
Discussion
This study confirmed the hypothesis that clozapine/NDMC ratio is strongly and negatively associated with working memory performance in patients with schizophrenia. This association was independent of age, gender, and symptom severity. We also did not find such a relationship with any other cognitive domain. Finally, we found a strong association between clozapine concentration and SAA but not between working memory performance and SAA.
Our findings are consistent with the results of two previous retrospective studies assessing the relationship between clozapine and NDMC concentrations and cognition. Our study confirms these preliminary findings in a well-characterized sample of adult patients with schizophrenia receiving clozapine monotherapy and recruited in a study designed to assess this relationship. Our study also shows that the association between clozapine/NDMC ratio and cognition is specific to working memory, as predicted by the role of cholinergic neurotransmission in cognitive function.
NDMC is a major metabolite of clozapine and is produced via
N-desmethylation of clozapine by CYP1A2, CYP2C19, and CYP3A4 (
29,
30). It has M
1 receptor agonist activity (
19) and potentiates hippocampal NMDA receptor currents through M
1 receptor activation (
20). NDMC could affect cognition through these interactions with NMDA receptor-dependent neuroplasticity. It could also improve cognition by increasing dopaminergic and cholinergic neurotransmission in the medial prefrontal cortex and hippocampus, both of which are blocked by an M
1 receptor antagonist (
31). Finally, NDMC could have cognitive effects by decreasing inhibitory synaptic transmission mediated by postsynaptic GABA
A receptors (
32). These effects could be further complicated by baseline dopaminergic tone in the frontal cortex as determined by each person’s unique characteristics, such as being affected by schizophrenia, having been exposed to antipsychotic medication for several years, and genetic makeup, such as having catechol-
O-methyltransferase (COMT) Val158Met polymorphism.
The effects of clozapine and NDMC on cognition via mechanisms mediated by the M
1 receptor have been demonstrated in animal studies. In one rodent study (
33), clozapine was found to impair performance on a working memory task while NDMC enhanced it. In the same study, the procognitive effects of NDMC were found to be reversed by an M
1 receptor antagonist. In a study in phospholipase C-β1 knockout mice known to have M
1 muscarinic receptor dysfunction (
34), behavioral deficits were reversed after administration of NDMC. Other studies have demonstrated positive behavioral effects of NDMC via muscarinic receptor neurotransmission activation (
35) or an NMDA receptor-dependent mechanism (
36). Still, in another rodent study, NDMC had no effects on the novel object recognition deficits induced by a hypoglutamatergic state, while clozapine caused further impairment (
37). Taken together, these studies and the present findings suggest that NDMC could have a procognitive effect, or it could merely offset the deleterious effect of clozapine. Furthermore, the opposing effects of clozapine and NDMC on cognition are probably the reason why clozapine and NDMC, separately, are not associated with cognition in patients with schizophrenia. In contrast, clozapine/NDMC ratio is a stronger predictor of cognition because it accounts for these opposing effects.
We did not find any significant relationship between clozapine/NDMC ratio and any cognitive domain other than working memory. We believe this is because the cholinergic system is robustly and selectively critical in performing tasks that depend heavily on complex attentional processes (
38). The MCCB working memory tests are highly dependent on complex attentional processes and therefore on cholinergic neurotransmission. The MCCB tests of other cognitive domains (e.g., episodic memory and reasoning and problem solving) are likely to depend on attentional processes, encoding of information, and short-term retrieval in episodic memory and planning and inhibitory mechanisms in problem solving, which are as dependent on other neurochemical systems as on the cholinergic system, and possibly more so.
As expected, SAA was strongly correlated with clozapine and NDMC concentrations. However, the association with NDMC concentration did not remain significant after we controlled for the association with clozapine concentration, which suggests that SAA is more reflective of clozapine curtailing the binding of NDMC. The absence of association between NDMC concentration and SAA after controlling for clozapine concentration could also be due to collinearity between clozapine and NDMC concentrations. Also as expected, we found that SAA was not associated with working memory performance. This is most likely due to the fact that there is no association between clozapine concentration alone and working memory performance and that SAA does not account for NDMC and its procognitive effects.
These findings seem to contradict those of a recent study in patients with schizophrenia in which SAA was inversely associated with both working memory performance and frontal gray matter density (
9). However, in that study, patients were receiving various conventional and atypical antipsychotic medications and some anticholinergic medications; only seven of the 47 patients were being treated with clozapine. Thus, in that study, cognition was most likely affected mainly by the antimuscarinic burden of medications and not likely by NDMC, and consequently SAA, reflecting this antimuscarinic burden, would be expected to be associated with cognition. This is consistent with a study in which SAA was not related to cognition in patients receiving only clozapine (
39) and with a follow-up study that included subjects receiving either clozapine or olanzapine (
40) in which SAA was negatively associated with frontal executive control. It is also consistent with the fact that the majority of subjects who demonstrated a lack of cognitive improvement associated with high SAA during cognitive training were receiving olanzapine rather than clozapine (
8). Taken together, these findings suggest that these other antipsychotics do not produce metabolites that could offset their anticholinergic burden and its negative impact on cognition.
Our study has some limitations. Because our design was cross-sectional, we did not assess the impact of changing clozapine/NDMC ratio on working memory. Such an intervention would be a logical follow-up to our study. While the MCCB was designed to assess the effects of interventions on cognition, it is possible that it is not sensitive enough to detect effects of clozapine and NDMC concentrations on cognitive domains other than working memory. Similarly, given the small size of our sample, we may have missed small effects. This limitation is partly offset by the homogeneity of our sample, which consisted exclusively of patients treated with clozapine monotherapy and a bedtime regimen. Still, replication in a larger sample is warranted.
Notwithstanding these limitations, our results have several potential implications both for future research and clinical practice. First, they highlight the importance of assessing the state of a target neurochemical system when attempting to intervene with agents that alter this system to enhance cognition. Our study specifically illustrates the need to take into account the effects of both a parent compound and its active metabolites when assessing a potential procognitive agent. Second, our study supports recent efforts to target M
1 receptor neurotransmission to enhance cognition: such agents could serve as adjunctive cognitive enhancers in the treatment of patients of schizophrenia. Finally, our study demonstrates the cognitive cost of a high clozapine/NDMC ratio. In fact, given the high anticholinergic burden associated with clozapine (
18), one would expect patients to be almost delirious. Our results suggest that NDMC offsets such high cognitive costs. This is an important consideration, given the recent interest in increasing the clozapine/NDMC ratio to increase the efficacy and tolerability of clozapine (
41). Conversely, it suggests that cognitive benefits could be obtained by reducing a high dosage of clozapine, given that the clozapine concentration is likely to decline before the NDMC concentration if
N-desmethylation is saturated (
42). Alternatively, an agent that inhibits the metabolism of NDMC but not that clozapine (or does so to a lesser extent) could possibly improve cognition without worsening psychotic symptoms.
Taken together, our findings suggest that the clozapine/NDMC ratio should be monitored routinely. This is particularly true for patients undergoing pharmacological or other clinical changes that affect clozapine metabolism or those in whom cognitive change is noticed. A cognitive decline that is associated with an increase in the clozapine/NDMC ratio should warrant an evaluation of what could have caused the increase and an adjustment of the cause or of the clozapine dosage.