Identification of vulnerability factors for depression ultimately may enable researchers to formulate illness interception strategies for predicting and preempting the development of mood disorders. In this study, we investigated whether a putative but treatable biological mechanism of depression, namely, amygdala activity during positively and negatively valenced autobiographical memory recall, constitutes a vulnerability factor for depression. We assessed this neuroimaging biomarker in currently depressed individuals and in nondepressed groups that are vulnerable to depression, based on their past personal or family history.
Recalling specific, affectively positive autobiographical memories is central to adaptive functioning (
1). This memory system has been hypothesized to operate in part to maintain a positive mood (
2), with positive memory recall specifically important for regulating emotional well-being (
3). In healthy individuals, autobiographical memory recall is biased toward positive events (
4,
5). In contrast, individuals with depression manifest a bias away from positive memories, such that relative to healthy controls, they are less likely to recall a positive memory in response to a positive or neutral cue word (
5), are more likely to recall positive memories that are overgeneral as opposed to specific (
5,
6), and demonstrate longer latencies to recall memories in response to positive cues (
7). This absence of a positive memory bias may contribute to the maintenance of the negative mood and impaired problem solving evident in depression (
8), and it is therefore a potential therapeutic target.
However, no correlation between autobiographical memory performance and depressive symptoms has been found (e.g.,
9), and research directly targeting autobiographical memory recall by having participants recall positive memories with the purpose of improving mood has proven it to be ineffective (
10). Therefore, while autobiographical memory impairment is a key feature of depression (
8), it may not be the impairment in autobiographical recall itself that directly relates to the maintenance of depressive symptoms. Rather, the presence of this biased recall in conjunction with an abnormality in regional brain function subserving salience and emotional processing may underlie this bias. Therefore, we aimed to test the hypothesis that a potential mechanism underlying emotionally biased autobiographical memory recall and the development of depression involves abnormal amygdala activity.
The amygdala influences the salience of stimuli or events (
11), and amygdala engagement is critical for emotional processing and responding to both negative and positive emotional stimuli (
12). This has been shown to be true for autobiographical stimuli (
13), with healthy adults exhibiting greater amygdala activity during positive relative to negative autobiographical recall (
14). With laboratory stimuli such as words and faces, the amygdala response in depressed individuals is “doubly dissociated” from that in control subjects, such that individuals with depression show exaggerated responses to negative stimuli and attenuated responses to positive stimuli (
15). As the amygdala provides information about the salience of stimuli (
11) and the salience of positive stimuli is reduced in depression (
15), individuals with depression may not find positive memories as compelling and therefore expend less effort in recalling them, with the result that they are easily distracted by salient negative episodes. To test this hypothesis, we examined whether the doubly dissociated pattern of amygdala activity would be evident during emotionally valenced memory recall, with increased amygdala activity and connectivity with regions important for maintaining salience (i.e., the dorsal anterior cingulate) during positive memory recall, and decreased activity but increased connectivity with regions associated with effort (i.e., dorsomedial prefrontal cortex) during negative memory recall in controls relative to individuals with depression.
We previously identified regional brain differences during positive and negative memory recall in healthy subjects compared with depressed participants, healthy subjects at high risk for developing depression based on having a first-degree relative with depression (
16), and individuals in full remission from depression (
9). In whole-brain group analyses, we found differential recruitment of medial temporal structures but not the amygdala. The amygdala is a small structure located near the basotemporal surface, where the temporal signal-to-noise ratio is lower than in most other brain regions (
17). Thus, significant activation may be missed by global voxel-wise analyses, which require conservative corrections for multiple comparisons (
18). Therefore, to increase power to detect differences in amygdala activity, we increased our sample size and performed a small-volume-corrected region-of-interest analysis on the previously reported data. To identify other regions that interact with the amygdala during emotional autobiographical memory, we conducted a functional connectivity analysis.
In addition, we further investigated whether abnormal amygdala activity combined with biased memory recall might be associated with the development of depression. To test this hypothesis, we included a currently depressed group as well as two vulnerable groups: remitted and high-risk individuals. Including these vulnerable groups would assist us in distinguishing whether biased memory recall and amygdala activity reflect state markers or scars of depression on the one hand or trait-like persistent markers of illness on the other. While there are few abnormalities in information processing readily discerned in vulnerable groups without necessitating a mood induction (
19), autobiographical memory recall is one such processing bias evident in vulnerable samples without prior mood induction (
8,
9,
20), making it especially suitable for testing our hypotheses.
Given the amygdala’s role in positive memory recall, the difficulties individuals with depression have in recalling specific positive memories, and the role of positive memory recall in adaptive functioning, we predicted that amygdala responses to positive memories would be related to symptom severity in depression, to residual symptoms in remitted depression, and to deficits in recalling positive specific memories in both depression groups. However, we previously observed that autobiographical memory impairment extends to high-risk individuals without depressive symptoms (
16), suggesting that this impairment interacts with some factor to lead to depression. Therefore, we did not expect any difference in amygdala activity between high-risk participants and controls. If this hypothesis were supported, it would suggest that amygdala hypoactivity to positive or hyperactivity to negative memories, in conjunction with the absence of a positive recall bias, may be causally related to the development and maintenance of depression, and that interventions targeting amygdala activity, such as real-time functional fMRI-based neurofeedback during emotional memory recall, may hold therapeutic potential (
21).
Method
Participants
A total of 160 medically healthy right-handed individuals 18 to 55 years old were recruited from the community and enrolled in one of four participant groups: psychiatrically healthy individuals with no first-degree relatives with a history of a mood disorder (N=60; control group), psychiatrically healthy individuals with a first-degree relative with depression (N=30; high-risk group), unmedicated individuals in a current major depressive episode according to DSM-IV-TR criteria (N=45; depressed group), and unmedicated individuals remitted from depression as defined by DSM-IV-TR criteria and with a Hamilton Depression Rating Scale (HAM-D) score <7 (N=25; remitted group) (
22). Participants underwent medical and psychiatric screening evaluations, which included the Structured Clinical Interview for DSM-IV Axis I Disorders (
23). Data from subsets of these participants have been reported previously (
9,
16,
24,
25), but this is the first report combining all samples.
Exclusion criteria included current pregnancy, general MRI exclusions, serious suicidal ideation, psychosis, major medical or neurological disorders, exposure to medication likely to influence cerebral function or blood flow within the past 3 weeks (3 months for the remitted group), and meeting DSM-IV-TR criteria for drug or alcohol abuse within the past year or for lifetime dependence (except nicotine). Additional exclusion criteria applied to the high-risk and control groups were current or past history of any major psychiatric disorder. After receiving a complete description of the study procedures, all participants provided written informed consent as approved by the Western Institutional Review Board. Participants received financial compensation for their participation.
Intelligence testing was conducted with the two-subtest (vocabulary and matrix reasoning) version of the Wechsler Abbreviated Scale of Intelligence (
26). Anxiety and depressive symptoms were rated using the 21-item HAM-D (
27), the State-Trait Anxiety Inventory (STAI) (
28), and the Profile of Mood States (POMS) (
29).
fMRI Autobiographical Memory Task
Details of the functional MRI (fMRI) data acquisition parameters and autobiographical memory task have been reported previously (
9,
16). Briefly, blood-oxygen-level-dependent (BOLD) fMRI was performed on a 3-T GE Discovery MR750 scanner and an eight-channel head coil. Participants were presented with an emotionally valenced or neutral cue word (20 positive, 20 negative, 20 neutral) and instructed to recall a past experience for 12 seconds and rate their retrieved memory on specificity and valence. Participants were instructed on the definitions of the standard memory categorizations of specific, categorical, extended, and semantic (
8,
30), and were able to provide and classify examples of each type before scanning was started. Autobiographical memory recall was compared with a semantic example generation condition in which participants were presented with an emotionally valenced or neutral cue word and instructed to think of seven examples from the category, then rate the ease with which they generated examples and report the number generated. Following each cue presentation and set of ratings, participants engaged in a riser detection task involving nonword letter strings as a control for visual input/attention.
Data Analysis
fMRI analysis was performed using AFNI (
http://afni.nimh.nih.gov/afni), and consisted of slice timing correction, within-subject realignment, coregistration between anatomical and functional images, spatial normalization to a common stereotaxic array (
31), and spatial smoothing (Gaussian kernel, 4 mm full width at half maximum). General linear model analysis was performed using AFNI’s 3dDeconvolve program. For each participant, the hemodynamic response to each event type was modeled as a boxcar function convolved with a canonical hemodynamic response function. The main effects of interest were cue word presentations that prompted positive specific memory recall, negative specific memory recall, example generation in response to positive cues, and example generation in response to negative cues. In addition to regressors modeling main effects and subject motion, each design matrix included regressors modeling rating selection and cue presentations in which other types of memories were recalled (categorical, extended, semantic). The nonword letter strings used as stimuli for the riser detection task were modeled as the baseline. A whole brain analysis of variance (ANOVA) was also performed to determine whether findings emerging from the present analysis replicate the patterns reported previously in other analyses of this sample (see the
data supplement that accompanies the online edition of this article).
We defined regions of interest for the left and right amygdala using Talairach amygdala masks within AFNI. For each participant, the 3dDeconvolve output was resampled and the amygdala mask applied (AFNI’s 3dROIstats) to calculate the percent signal change within each region of interest for specific positive memories versus positive example generation and specific negative memories versus negative example generation.
The resulting percent signal change values were entered into a repeated-measures ANOVA with the repeated measure of valence (positive, negative) and amygdala region of interest (left, right), and the between-group factor of group (control, high-risk, depressed, remitted) using SYSTAT, version 13 (Systat Software, San Jose, Calif.). We decided a priori to perform Pearson correlations between amygdala activity, mood ratings, and memory performance to detect associations between amygdala activity, mood, and memory overgenerality. The threshold criterion for significance was set at Bonferroni-corrected p value of 0.05, applied for each post hoc group comparison and correlation (e.g., all control group versus high-risk group tests corrected for 36 comparisons [all variables in
Table 1 plus four amygdala variables] and all control versus high-risk group correlations corrected for 50 multiple comparisons [25 variables in
Table 1 excluding nonspecific memories, correlated with amygdala activity during positive and negative memory recall separately]). Potential group differences in age, IQ, memory performance, and depression and anxiety ratings were assessed using a one-way ANOVA.
For the generalized linear model-based functional connectivity analysis, the data were low band pass filtered at 0.10 Hz and the left amygdala seed was anatomically defined. The time course of the mean BOLD signal from the seed during positive and negative memory recall were used, separately, as the stimulus regressor. The generalized linear model for each run also included six motion parameters. The generalized linear model based R2 statistics were converted to correlation coefficients and converted to z scores using the Fisher r-to-z transformation. AFNI’s 3dANOVA was used to determine how the functional connectivity pattern differed between groups. The significance threshold was set at corrected p value of 0.05 (3dClustSim cluster size >30 voxels, voxel p value <0.005).
Results
Demographic and Clinical Characteristics
The upper section of
Table 1 presents means and standard deviations for demographic and mood ratings for each group (for clinical characteristics of the depression groups, see Table S1 in the online
data supplement). The groups did not differ in age, IQ, or gender distribution but differed significantly in mood ratings, with depressed participants reporting significantly different ratings (indicating more negative overall mood) compared with the other groups (compared with the control group: corrected p values, <0.001; d values, >1.24; high-risk group: corrected p values, <0.001; d values >1.07; remitted group: corrected p values, <0.002; d values >0.85). While remitted participants had scores within the healthy range on all measures, their HAM-D scores, trait anxiety ratings, POMS depression, fatigue, and confusion subscale ratings, and total POMS scores were elevated relative to both healthy groups (compared with the control group: corrected p values, <0.02; d values >0.61; high-risk group: corrected p values, <0.02; d values >0.66). The high-risk and control groups did not differ significantly from each other on any clinical measure.
The POMS and STAI were administered immediately before and after completion of the fMRI task, and the associated omnibus tests are reported in the middle section of
Table 1. No rating in the control or high-risk group changed significantly from pre- to postscan assessment, whereas POMS depression subscale and STAI trait anxiety ratings decreased significantly in both the depressed and remitted groups (corrected p values, <0.01 and <0.009, respectively). The depressed and remitted groups had significantly greater decreases in these measures compared with both the control group (compared with the depressed group: corrected p values, <0.005, d values, >0.52; remitted group: corrected p values, <0.004; d values >0.57) and the high-risk group (compared with the depressed group: corrected p values, <0.03; d values, >0.47; remitted group: corrected p values, <0.03; d values, >0.43). These change measures did not differ significantly between the remitted and depressed groups or between the control and high-risk groups.
Autobiographical Memory Recall
The lower section in
Table 1 lists the percentage of each memory type recalled for each group and a breakdown of the percentages of specific memories recalled for each valence, as specific memory valence is the focus of the study. We replicated the overgenerality effect; control subjects recalled more specific and fewer categorical memories compared with all other groups (compared with the high-risk group: corrected p values, <0.001; d values >1.07; remitted group: corrected p values, <0.02; d values >1.67; depressed group: corrected p values, <0.001; d values >1.47), and these other groups did not differ from each other. No other memory categorization differed between groups. When examining the percentages of specific memories rated as positive, negative, and neutral, we found that the control and high-risk group had more positive specific memories than either the remitted group (compared with the control group: corrected p=0.01; d=0.57; high-risk group: corrected p=0.04; d=0.48) or the depressed group (compared with the control group: corrected p<0.001; d=0.99; high-risk group: corrected p=0.003; d=0.92), and the depressed group had significantly more negative specific memories compared with the control group (corrected p=0.001; d=0.82).
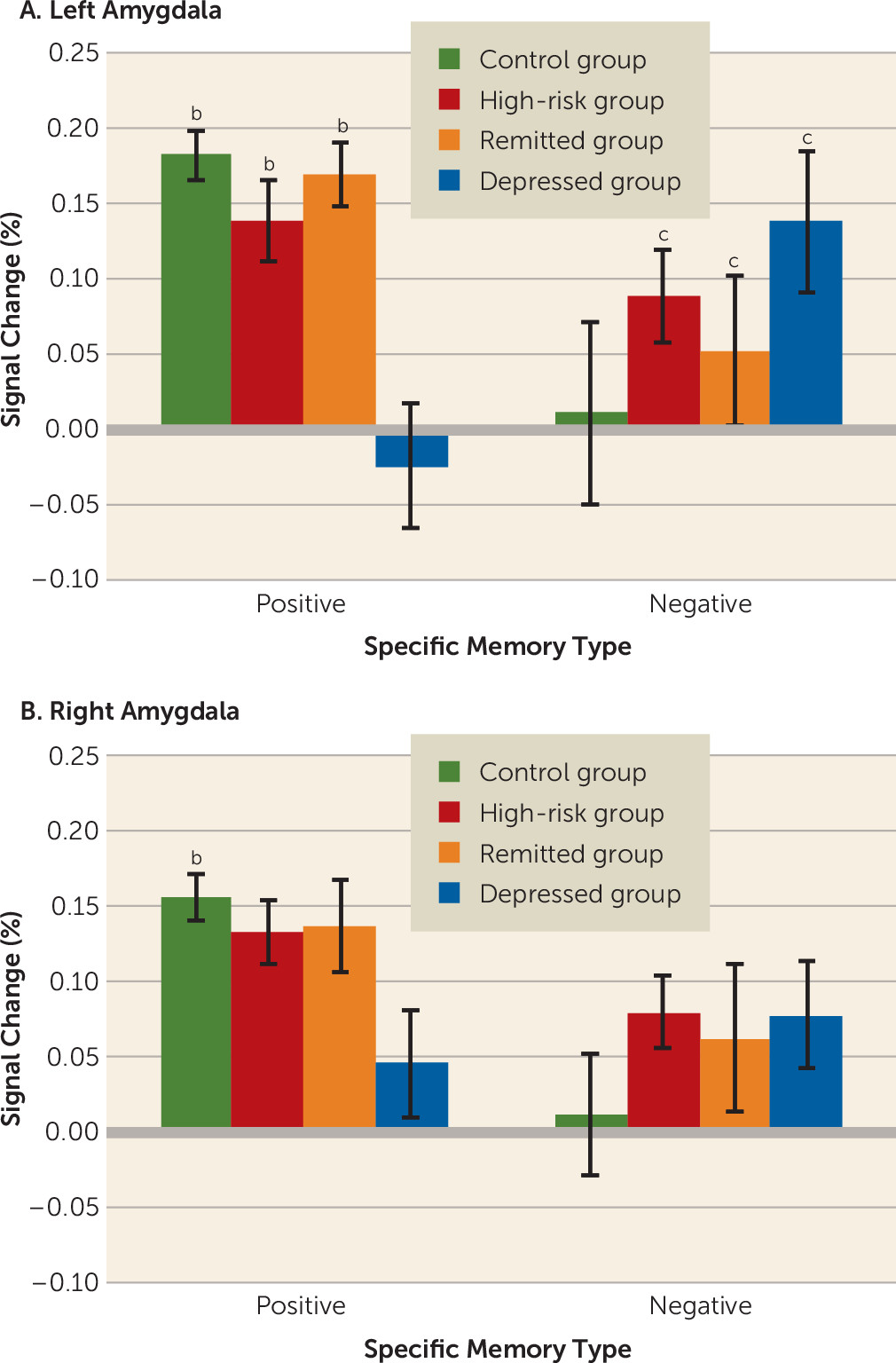
Amygdala BOLD Activity
Figure 1 shows the percent signal change within the left and right amygdalae during positive memory recall relative to positive example generation and negative memory recall relative to negative example generation. There was a significant region of interest-by-valence-by-group interaction (F=7.20, df=3, 156, p<0.001) in the left amygdala, with depressed participants exhibiting decreased activity in response to positive specific memory recall relative to the other groups (compared with the control group: corrected p=0.001; d=0.92; high-risk group: corrected p=0.004; d=0.71; remitted group: corrected p=0.001; d=0.89) and the other groups not differing from each other. When left amygdala activity was examined during negative specific memory recall, the control group had decreased activity relative to all of the other groups (compared with the high-risk group: corrected p=0.002; d=0.23; remitted group: corrected p=0.02; d=0.11; depressed group: corrected p<0.001; d=0.33), which did not differ from each other. In the right amygdala, the same pattern of activity was observed, but the only difference that reached statistical significance was for greater activity during positive memory recall versus positive example generation in the control group compared with the depressed group (corrected p=0.001; d=0.58). An additional analysis confirmed that this was the case for both the new participants and those included in previous studies (see the online
data supplement).
Correlation Between Amygdala Response, Mood, and Memory Performance
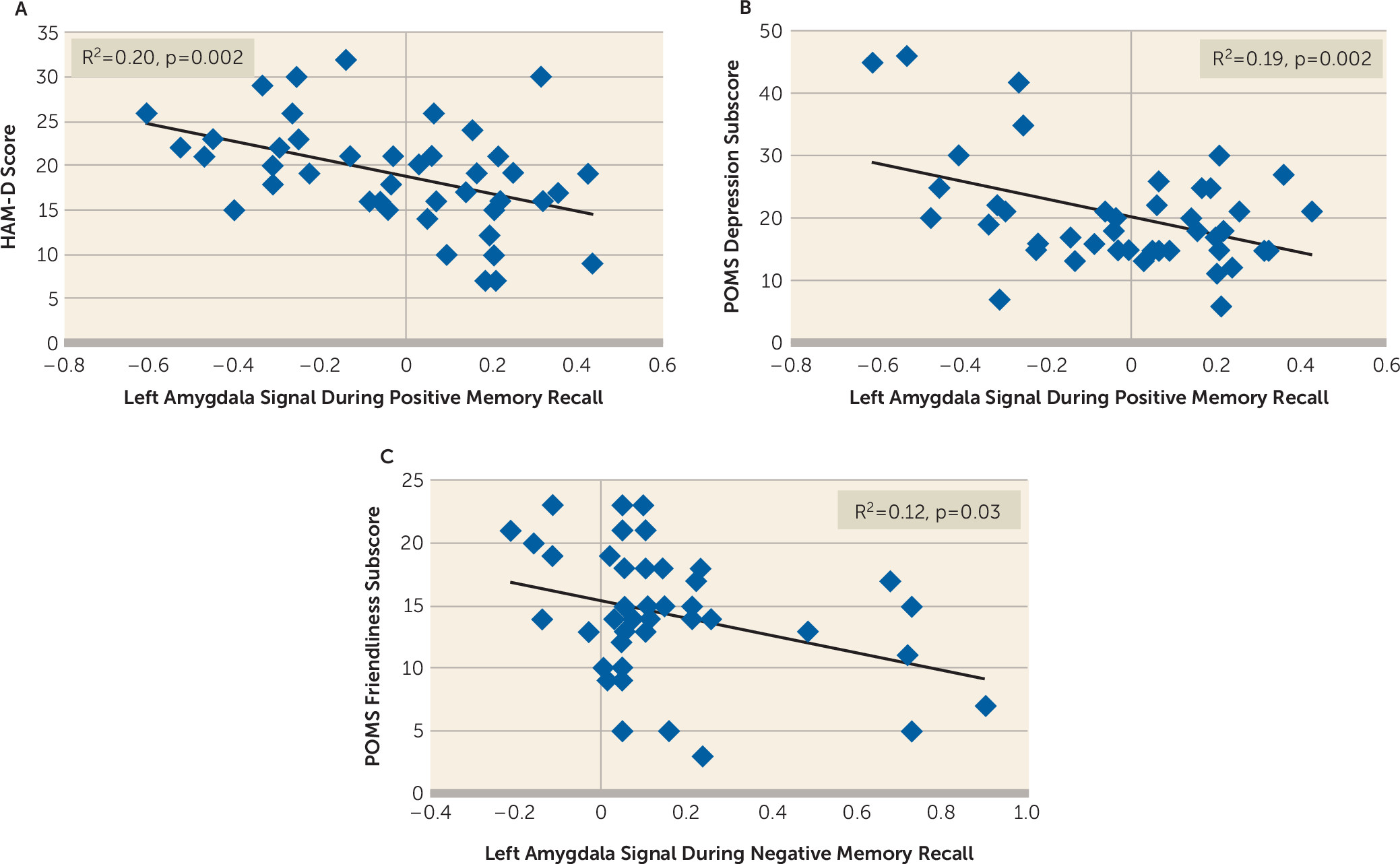
Because of small variance in scores and memory performance in the healthy groups, we examined correlations separately for each group (
Figure 2). There was a significant inverse correlation in depressed participants, with higher left amygdala activity during positive memory recall associated with lower HAM-D score (r=−0.45, corrected p=0.002) and POMS depression subscale score (r=−0.43, corrected p=0.004). No significant correlation was found in the other groups. During negative memory recall, one correlation remained significant after applying corrections for multiple testing: an inverse relationship in the depressed group between left amygdala activity during negative memory recall and POMS ratings of friendliness (r=−0.34, corrected p=0.03).
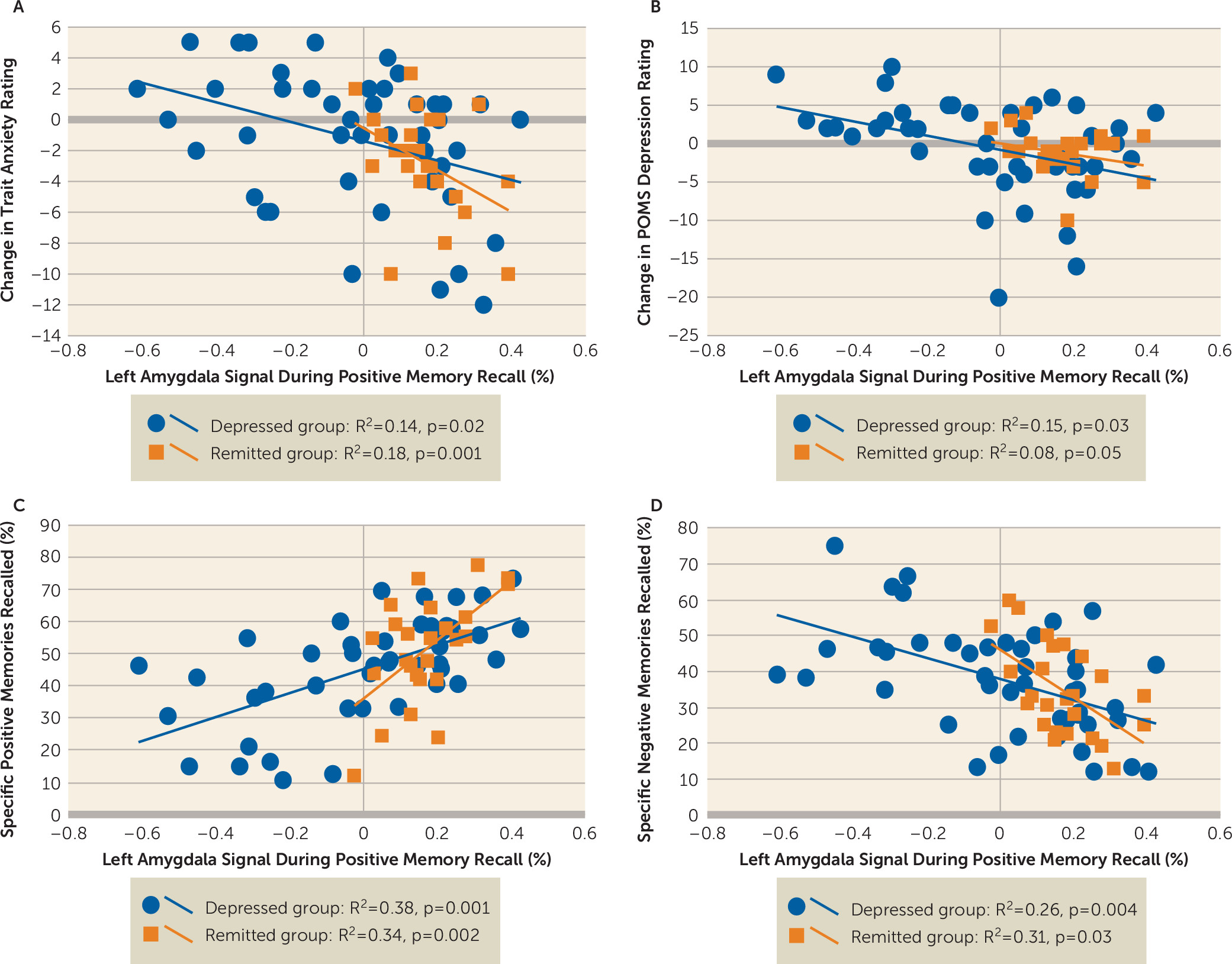
We also examined the relationship between amygdala activity during memory recall and changes in mood ratings between pre- and postscan assessments (
Figure 3). We found no significant correlation in the healthy groups. However, the depression groups exhibited a significant inverse correlation between left amygdala activity during positive memory recall and changes in STAI trait anxiety ratings (depressed group: r=−0.37, corrected p=0.02; remitted group: r=−0.42, corrected p=0.001) and POMS depression scores (depressed group: r=−0.39, corrected p=0.03; remitted group: r=−0.28, corrected p=0.05).
Finally, we examined the relationship between amygdala activity during memory recall and the percent of specific memories recalled for each valence and overall percent of specific memories recalled (
Figure 3). There was no significant correlation in the healthy groups. However, in the depression groups, there was a significant correlation between left amygdala activity during positive memory recall and the percent of positive specific memories recalled (depressed group: r=0.61, corrected p=0.001; remitted group: r=0.59, corrected p=0.002) and an inverse correlation between left amygdala activity during positive memory recall and the percent of negative memories recalled (depressed group: r=−0.51, corrected p=0.004; remitted group: r=−0.56, corrected p=0.03).
Amygdala Functional Connectivity
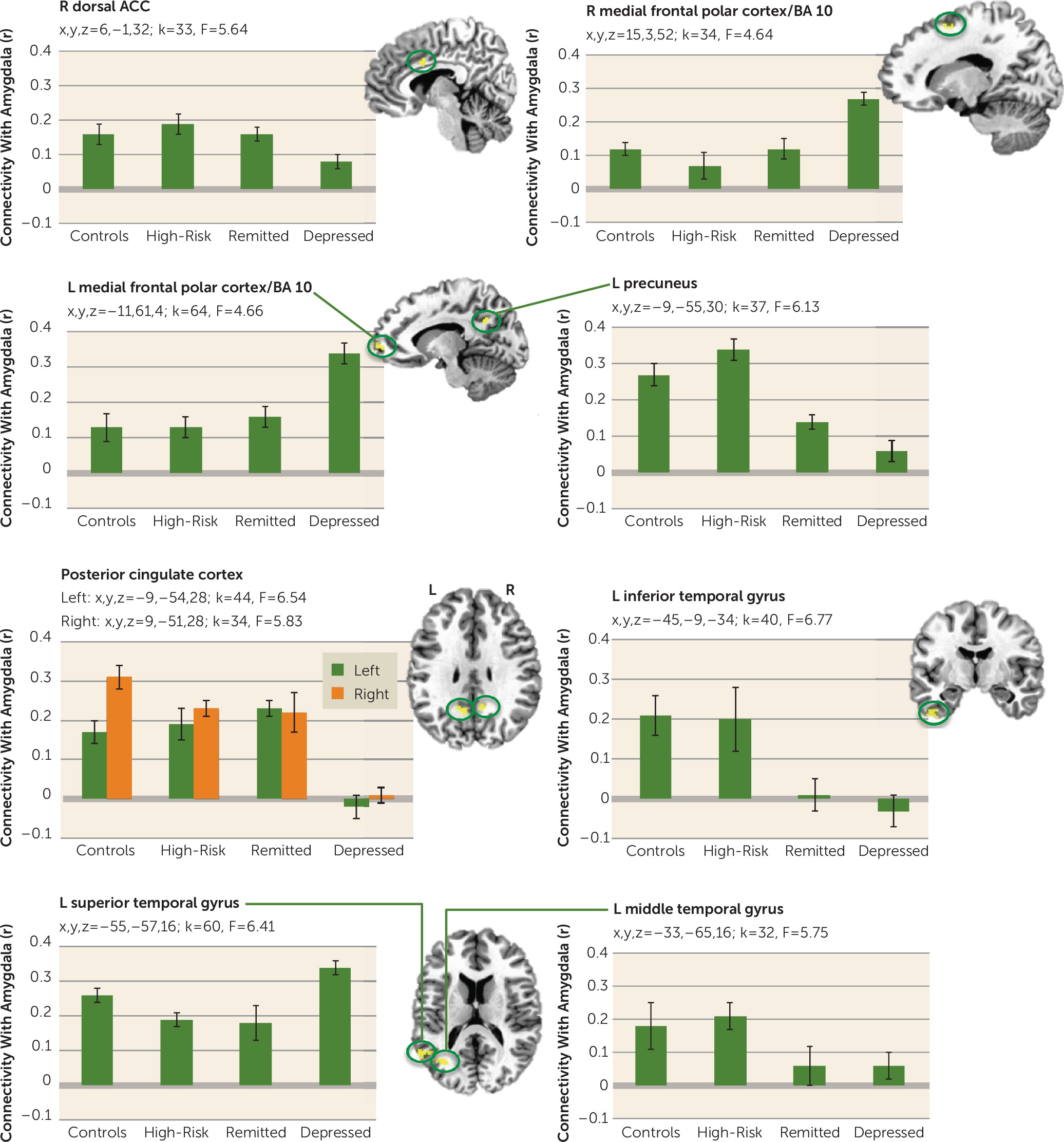
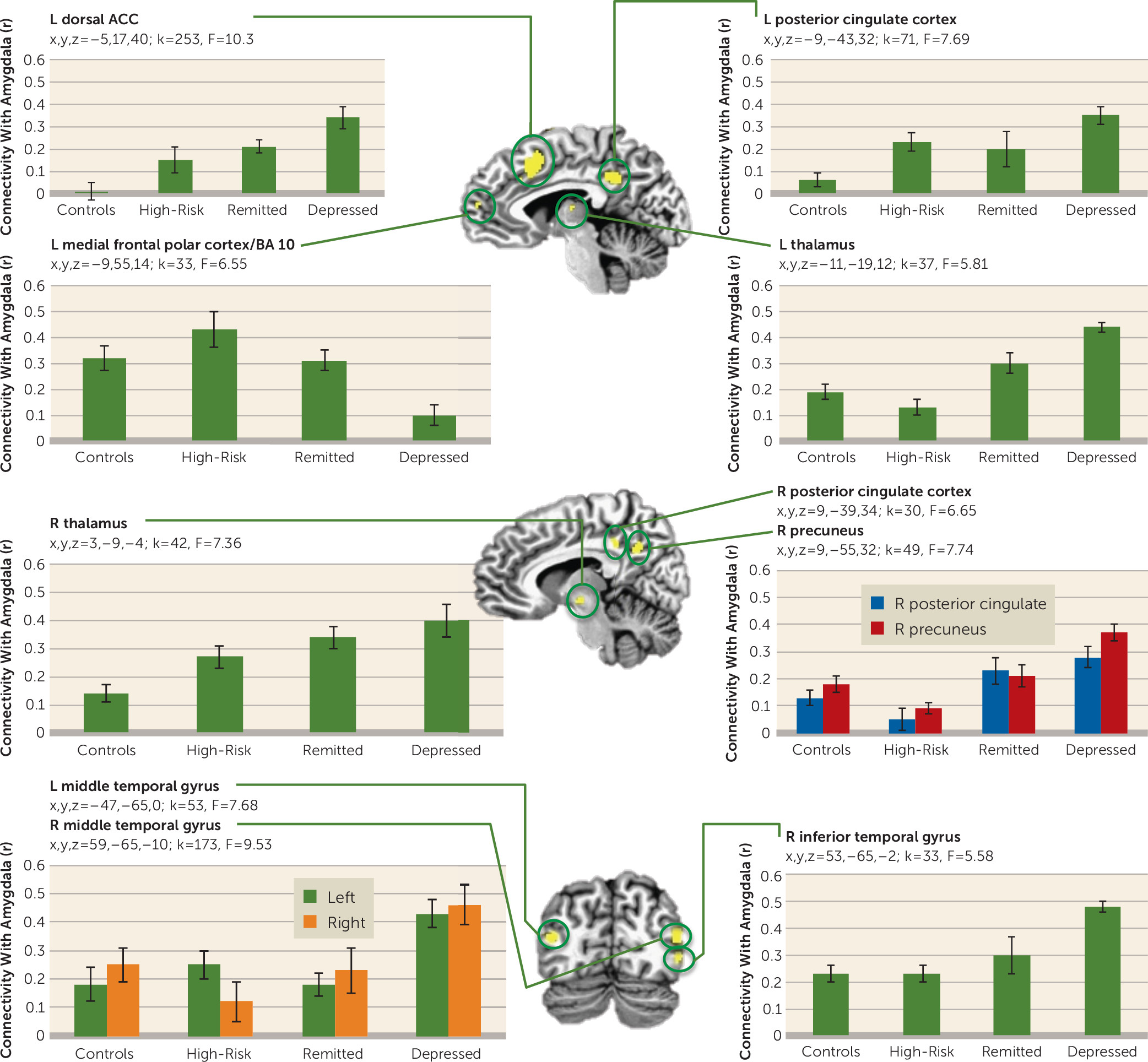
Figures 4 and
5 show regions where the groups differed in functional connectivity with the amygdala during positive and negative memory recall. During positive memory recall (
Figure 4), the depressed group had less amygdala connectivity with the right dorsal anterior cingulate cortex and the left and right posterior cingulate cortex than all of the other groups (whose amygdala connectivity was similar to each other). Conversely, the depressed group had higher amygdala connectivity with the left and right medial frontal polar cortex and the left superior temporal gyrus than the other groups. Amygdala connectivity with the left precuneus and the left middle and inferior temporal gyrus was greater in both healthy groups relative to each of the depression groups.
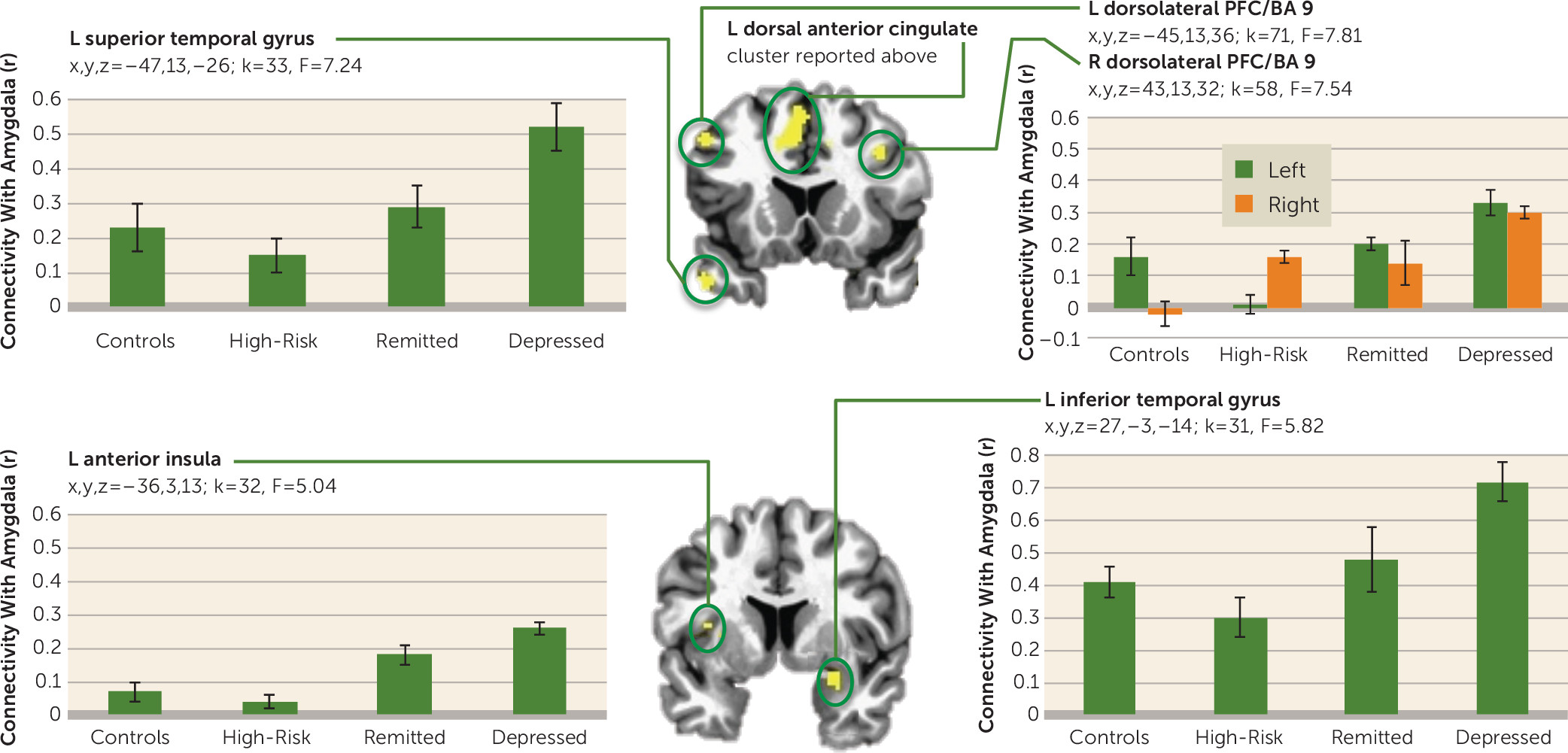
During negative memory recall (
Figure 5), amygdala connectivity was increased in the depressed group relative to the other three groups with all regions in
Figure 5 (left dorsal anterior cingulate cortex; superior temporal gyrus and insula; left and right dorsolateral prefrontal cortex, posterior cingulate cortex, thalamus, and middle temporal gyrus; and right precuneus, inferior temporal gyrus, and amygdala) except the left medial frontal polar cortex, which showed lower amygdala connectivity in the depressed group. For the regions that showed the greatest amygdala connectivity in the depressed group, the connectivity was lowest in either the control group or the high-risk group, with the remitted group showing lower amygdala connectivity than the depressed group but higher than at least one of the healthy groups.
Discussion
This study demonstrates abnormal patterns of amygdala activity in depressed compared with control, high-risk, and remitted participants as they engage in autobiographical memory recall. While left amygdala activity was elevated in the depressed, remitted, and high-risk groups relative to the control group during negative specific memory recall, activity was lower in the depressed group compared with the other groups during positive memory recall.
The results suggest that amygdala hyperactivity during negative memory may be a trait-like marker of depression, as both vulnerable groups showed activity similar to that of the depressed group, and higher than that of the control group. In contrast, amygdala hypoactivity and reduced functional connectivity with regions of the salience and self-referential networks during positive specific memory recall may constitute a state marker of depression that manifests during active (symptomatic) illness and returns to normal with symptom remission. However, the present study employed a cross-sectional design, so the imaging differences may have been the result of unidentified dissimilarities between groups. Definitive conclusions regarding state/trait markers thus require longitudinal studies in which the same participants undergo scanning in different mood states.
The finding that depressed participants with lower self-reported and clinician-rated depressive symptoms had higher left amygdala activity during positive memory recall further suggests that amygdala hypoactivity to positive stimuli is clinically relevant. To the extent that the amygdala is important in maintaining the salience of an emotional cue (
11), we hypothesize that low amygdala reactivity to positive memories reduces their salience and thus decreases the likelihood or frequency of their being recalled. Notably, amygdala connectivity with regions that form the salience network (dorsal anterior cingulate cortex) (
32) and those implicated in self-referential processing (precuneus, posterior cingulate) (
33) was reduced in the depressed group relative to all other groups during positive memory recall, while amygdala connectivity with regions involved in effortful reasoning within the domain of social cognition (frontal polar cortex) (
34) was increased in the depressed group relative to the other groups, further supporting our hypothesis that positive memories are not rendered salient or personally relevant during positive memory recall and therefore patients expend less effort recalling them (and that abnormalities in positive processing are state markers of depression). The inverse correlation between amygdala activity during positive memory recall and the percent of negative memories recalled further suggests that patients who are able to recruit the amygdala during positive memory recall manifest an adaptive emotional processing bias shifted toward the positive (and away from the negative) valence.
Furthermore, we replicated the overgenerality effect, with the depressed (
8) and vulnerable groups (
9,
16) recalling fewer specific and more categorical memories relative to the control group. The greater statistical power of the present study revealed that depressed participants had more negative specific memories than control subjects in addition to fewer positive memories. There was a nominal trend in this direction in our previous studies (
16,
35). These data suggest that depression is characterized by a bias toward specific negative memories. Clinically, if individuals spontaneously remember specific negative events but only have vague recollections of their positive experiences, they may spend more time contemplating negative than positive events (
36), or sustain a cognitive style involving rumination on negative events (
37). Including measures of rumination would enable more direct tests of this hypothesis. However, the hypothesis that negative events are more salient and easier to recall for depressed relative to nondepressed individuals is further supported by the findings that amygdala connectivity during negative memory recall was abnormally increased with regions of the salience network (dorsal anterior cingulate cortex and anterior insula) (
32) and those involved in self-referential processing (precuneus, dorsolateral prefrontal cortex) (
33,
38), but decreased with regions involved in effortful processing (frontal polar cortex) (
34) in the depressed and remitted groups.
In both currently and formerly depressed participants, ratings of depression and anxiety decreased after completion of the autobiographical memory task and were associated with higher amygdala activity during positive memory recall. No such relationship was found between ratings and amygdala responses during negative specific memory recall. The significant decrease in anxiety ratings seen in both depression groups was evident only for the trait measure of anxiety on the STAI; no differences were found on the STAI state anxiety measure. Test-retest reliability on the STAI trait measure is 0.89 over a 2-month interval (
28), which suggests that this indeed reflects a stable measure of anxiety. That both depression groups showed a significant decrease on this “stable” measure suggests that completing the task resulted in these individuals reevaluating their life in a less negative manner, consistent with anecdotal reports from depressed participants that they enjoyed performing the task. That this change was correlated with amygdala activity during positive memory recall suggests that those individuals with depression best able to recruit their amygdala during positive memory recall experience the most emotional benefit, and that it is the amygdala response to positive stimuli that is clinically relevant (as no correlation was found between mood change and amygdala responses to negative memories).
We have begun to further explore the hypothesis that amygdala hypoactivity is causally related to depression by using real-time fMRI neurofeedback to train depressed participants to up-regulate their amygdala response during positive memory recall. The results (
21) supported our hypothesis: successfully learning to up-regulate the amygdala response to positive memories via real-time fMRI neurofeedback decreased STAI ratings of anxiety and POMS depression ratings and increased ratings of happiness in depressed participants. These results were observed after a single session of amygdala neurofeedback, suggesting beneficial effects for individuals in an acute depressive episode. Further testing of the clinical efficacy of this procedure as an intervention for depression is under way (ClinicalTrials.gov identifier: NCT02079610).
Conclusions
We have presented evidence that amygdala responses to emotional stimuli in depression are doubly dissociated from those in nondepressed individuals by virtue of an increased response to negative memories along with increased functional connectivity to regions implicated in salience and self-referential processing, and a decreased response to positive memories along with decreased functional connectivity in salience and self-referential processing regions. Amygdala hyperactivity to negative memory recall may thus constitute a trait-like marker of depression, as it is evident in at-risk groups. In contrast, amygdala hypoactivity to positive stimuli is only observed during the active illness state and is related to depressive symptoms and the lack of a normative positive processing bias during memory recall, potentially due to positive stimuli not recruiting the amygdala to a sufficient extent to render them sufficiently salient to generate a specific memory. These data suggest that treatments directly targeting amygdala hypoactivity to positive stimuli hold therapeutic potential for individuals with depression.