The term “reactive aggression” has been used to describe retaliatory actions in response to perceived threat, frustration, or provocation (
1). Youths with disruptive behavior disorders (DBD), such as conduct disorder and oppositional defiant disorder, commonly display maladaptively higher levels of reactive aggression than youths without psychopathology (
2,
3). Such high levels of aggression typically occur in the context of other forms of antisocial behavior (
4), as well as decision-making deficits (
5). In this study, we examined the neural correlates of retaliatory actions in healthy youths and youths with DBD.
Previous research has examined such neural correlates based on response to social provocation in variants of the ultimatum game (
6). In one variant, a computer-simulated partner proposes to divide resources with participants. Participants accept or reject the division. Participants are more likely to reject an unfair offer if the partner could have offered a fair allocation, but the magnitude of this effect is reduced in incarcerated individuals (
7,
8). Another ultimatum game variant involves participants choosing whether to “punish” the partner by spending money to remove the partner’s money. Such behaviors expressed in this variant serve to model retaliatory behavior in participants. Notably, increasing levels of retaliatory behavior are associated with increased activity in regions implicated in reactive aggression in animal studies (amygdala, periaqueductal gray) (
9–
12). Furthermore, neuroimaging studies implicate an inverse relationship between ventromedial prefrontal cortex activation and amygdala/periaqueductal gray response to basic threats (
13) and provocation (
9,
11). The ventromedial prefrontal cortex is thought to be critical for representing expected value (i.e., the subjective reward value that an individual associates with an action or object following learning [
14]). Decreased ventromedial prefrontal cortex activation may reflect the representation of the diminishing expected value of increasing levels of retaliation, as increasing retaliation leads to diminishing monetary gains in the ultimatum game (
9,
11). In threat induction paradigms, the increasing proximity of threat is associated with a diminishing likelihood of escape (and an increased likelihood of punishment); the value of future behavioral options diminishes as threat increases in proximity (
13). We have suggested (
15) that the increased risk for reactive aggression in patients with DBD reflects increased activity in threat response regions and/or dysfunction in ventromedial prefrontal cortex modulatory activity.
However, youths with DBD are heterogeneous. Some show elevated levels of callous-unemotional traits (DBD/+CU) (i.e., decreased guilt/empathy—referred to in DSM-5 as “with limited prosocial emotions”), while others do not (DBD/–CU). Youths with DBD/+CU and DBD/–CU have distinct developmental trajectories and neurobiology (
15,
16). Both groups, however, show elevated levels of reactive aggression (
3). The neurobiology of reactive aggression may be different for each group. We have hypothesized that youths with DBD/–CU show increased risk for reactive aggression because of heightened responsiveness of basic threat systems (amygdala/periaqueductal gray); the suggestion is that this increased responsiveness increases the probability that a given level of provocation will initiate fighting rather than flight or freezing (
15). There is evidence that youths with DBD/–CU show an increased amygdala response to fearful expressions, whereas those with DBD/+CU show a decreased amygdala response (
17–
19). Alternatively, there may be common neurobiological underpinnings of reactive aggression, which we have hypothesized reflect dysfunction in the modulatory role of the ventromedial prefrontal cortex in representing an action’s expected value (
15). Expected value representation is disrupted in patients with DBD irrespective of level of callous-unemotional traits (
5,
20). In the context of an ultimatum game, we expect that youths with DBD will fail to appropriately represent the diminishing value of outcomes as retaliation increases, leading to an increased propensity to retaliate. Here, we examine these possibilities through an investigation of retaliatory behavior and its neural underpinnings in the context of an ultimatum game.
This study tested four predictions: First, retaliatory propensity, measured by mean levels of punishment during the ultimatum game, would have a significantly greater association with reactive than proactive aggression. Second, the increase in amygdala/periaqueductal gray activity when retaliating would be exaggerated in youths with DBD/–CU relative to healthy youths, and the level of activity in these regions would be positively associated with reactive aggression. Third, the typical decrease in ventromedial prefrontal cortex activity when retaliating and in ventromedial prefrontal cortex-amygdala functional connectivity would both be attenuated in youths with DBD/–CU and DBD/+CU relative to healthy youths. Fourth, the level of attenuation of modulated response and the connectivity during high provocation/retaliation in the ventromedial prefrontal cortex would be negatively associated with retaliatory propensity.
Method
Participants
A total of 56 youths 10–18 years of age (mean=14.74 years, SD=2.13), 23 of them female, participated: 30 youths with DBD and 26 healthy youths. Consistent with previous work (
17), a median split on Inventory of Callous-Unemotional Traits score (median=43.5) was utilized to form DBD/+CU (mean=46.8, SD=3.57) and DBD/–CU (mean=33.9, SD=6.38) groups. The groups did not differ significantly in age, IQ, or gender composition (
Table 1). Half of the youths with DBD (N=15) were comorbid for attention deficit hyperactivity disorder (ADHD), and medications could not be withheld during scanning for 23.3% (N=7) of the DBD group. Youths were recruited from the community. After receiving a complete description of the study, children and parents provided written informed assent or consent. The NIH Institutional Review Board approved the study. Inclusion and exclusion criteria are listed in the supplemental methods section of the
data supplement that accompanies the online edition of this article.
Study Measures
Inventory of Callous-Unemotional Traits.
This 24-item parent-report scale (
21), designed to assess callous-unemotional traits in youths, was derived from the callous-unemotional scale of the Antisocial Process Screening Device (
22), which has been used in various youth samples. The construct validity of the Inventory of Callous-Unemotional Traits has been supported in community and juvenile justice samples (
23,
24).
Reactive/Proactive Aggression Rating Scale.
Parents completed this six-item measure (
1). Dodge and Coie (
1) found that a three-item reactive aggression scale and a three-item proactive aggression scale yielded the best-fitting model. This measure was found to be useful in distinguishing aggression subtypes in children (
1) and adolescents (
25).
The social fairness game.
Participants were presented with a version of the ultimatum game, the social fairness game, which has been described elsewhere (
11) (
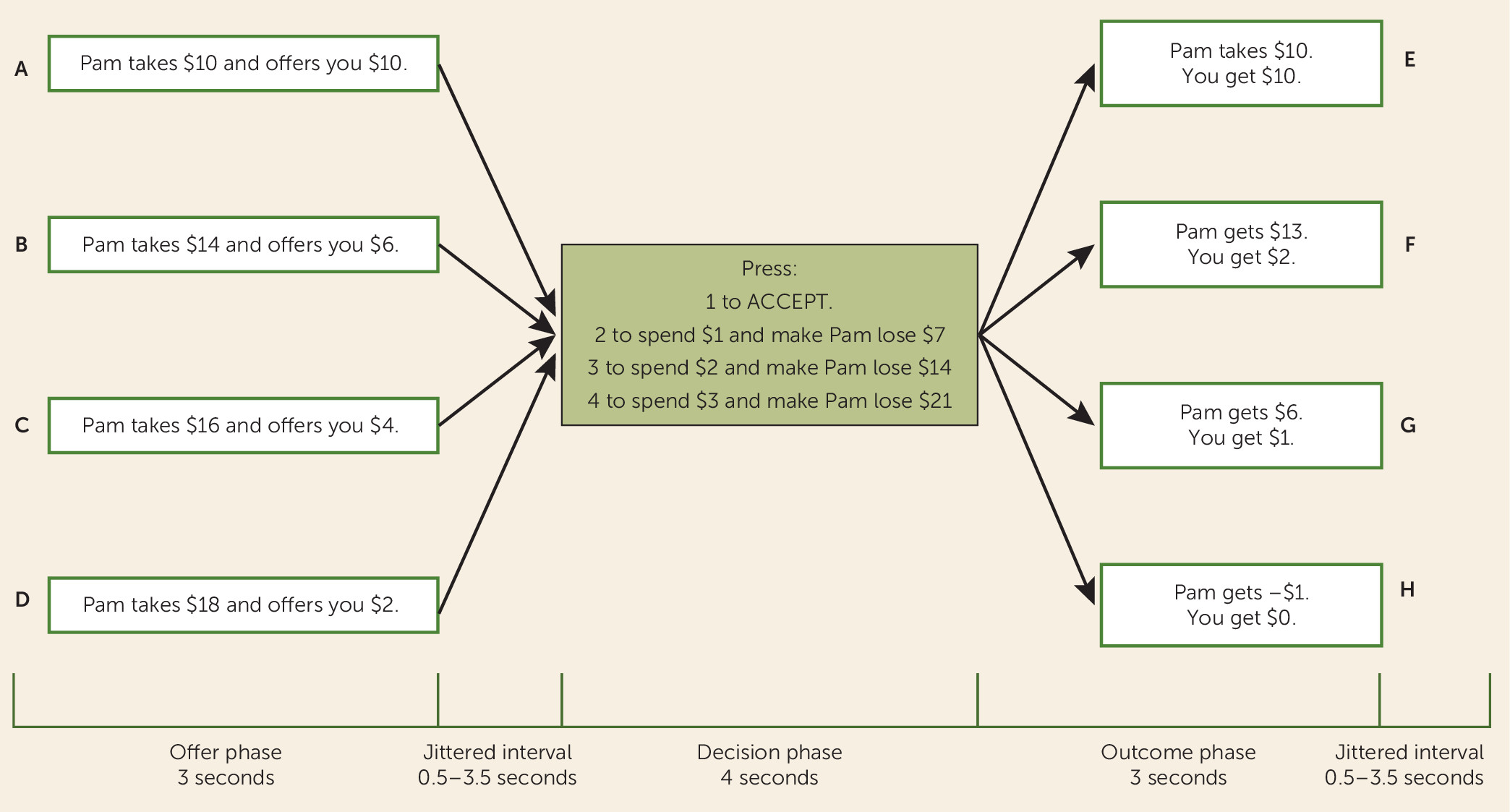
Figure 1; see also the online
data supplement). Participants are offered either a fair ($10 to participant; $10 to partner) or an unfair ($6/$4/$2 to participant; $14/$16/$18 to partner) division of a $20 pot. Participants could then either accept the offer or reject it and, by spending $1, $2, or $3, punish the partner; each punishment dollar spent by the participant caused the partner to lose $7 from the $20 pot (
Figure 1). Retaliatory propensity was defined as the participant’s average punishment level.
MRI Parameters and Preprocessing
Participants were scanned using a 3-T GE scanner, and data were analyzed using AFNI (
26). Specific parameters have been reported elsewhere (
11) and are also listed in the online
data supplement.
General Linear Model Analysis
The model involved six motion regressors and the following task regressors: indicator functions for the offer phase, the decision phase, the outcome phase, the offer phase multiplied by offer unfairness (scored as 0 [fair: $10 kept and $10 offered to partner], 8 [unfair: $14/$6], 12 [unfair: $16/$4], 16 [unfair: $18/$2]), and the decision phase multiplied by the sample average punishment level at each level of unfairness (average response to $10/$10, 1.176; to $14/$6, 2.358; to $16/$4, 2.726; to $18/$2, 3.230). All regressors were convolved with a canonical hemodynamic response function from the onset of the offer, decision, and outcome phases to account for the slow hemodynamic response. In accordance with findings that normalization of brain volumes from age 7–8 onward does not introduce major age-related distortions in localization or time course of the blood-oxygen-level-dependent (BOLD) signal in event-related functional MRI (fMRI) (
27,
28), the participants’ anatomical scans were individually registered to the Talairach and Tournoux atlas (
29). Participants’ functional echo planar image data were then registered to their Talairach anatomical scans. Linear regression modeling was performed using the five regressors described earlier, plus regressors to model a first-order baseline drift function. This produced beta coefficients and associated t statistics for each voxel and regressor.
fMRI Data Analysis
A three (diagnosis: DBD/+CU, DBD/–CU, healthy) by two (task phase: offer phase, decision phase) repeated-measures analysis of variance (ANOVA) was conducted on the modulated regression coefficients. The AFNI ClustSim program was used to establish a family-wise-error-corrected threshold (594-mm
3 clusters at p=0.005, corrected to p=0.05) for a whole-brain analysis. Because of their small size and/or theoretical importance, small-volume corrections for multiple comparisons were calculated for the amygdala, periaqueductal gray, and ventromedial prefrontal cortex. The amygdala small-volume correction, calculated using an anatomically defined mask (Eickhoff-Zilles Architectonic Atlas: 50% probability) (
30), yielded a threshold of 162 mm
3 at an initial significance threshold of 0.02. As anatomically defined masks were not available in AFNI, small-volume corrections for the periaqueductal gray and ventromedial prefrontal cortex were calculated using 10-mm spheres centered on the peak coordinates from previous work (periaqueductal gray (x, y, z): 3, −23, −4; ventromedial prefrontal cortex: −4, 36, −5) (
13) and yielded a threshold of 248 mm
3 at an initial significance threshold of 0.02 for both regions. For these analyses, average percent signal change was measured across all voxels within each region of interest generated from the functional masks, and post hoc testing was conducted in SPSS (IBM, Armonk, N.Y.).
Generalized psychophysiological interaction analyses (
31) were conducted to investigate differences in functional connectivity associated with DBD during the ultimatum game. As a parametric modulation strategy is incompatible with functional connectivity analyses, functional connectivity was compared using two three (diagnosis: DBD/+CU, DBD/–CU, healthy) by two (provocation level: low [fair/accept], high [unfair/punished]) by two (task phase: offer phase, decision phase) repeated-measures ANOVAs. The anatomically defined amygdala masks mentioned above were seed regions. (See the online
data supplement for details.)
Results
Behavioral Results
The relationship between retaliatory propensity (average punishment on the ultimatum game) and reactive aggression was significantly stronger than the relationship between retaliatory propensity and proactive aggression (whole group: r=0.26 and r=0.04, respectively; Steiger’s Z=2.13, p=0.03; patients with DBD only: r=0.37 and r=−0.13, respectively; Z=2.62, p<0.01). Additionally, among youths with DBD, there was a significantly stronger relationship between time to retaliation and reactive aggression relative to proactive aggression (r=−0.59 and r=−0.25, respectively; Z=2.00, p<0.05). Callous-unemotional traits among the youths with DBD were significantly related to proactive (r=0.45, p=0.01), but not reactive aggression (r=0.19, p=0.33), although these correlations were not significantly different (Z=1.44, p=0.15).
A four (offer unfairness: $10/$10, $14/$6, $16/$4, $18/$2) by two (diagnosis: DBD, healthy) repeated-measures ANOVA was conducted on the choice data (i.e., level of punishment selected: $0, $1, $2, $3). A significant main effect of offer was observed (F=420.93, df=3, 54, p<0.01). Participants increased retaliation as offer unfairness increased. Neither the main effect of diagnosis nor the unfairness level-by-diagnosis interaction was significant. However, a significant quadratic unfairness level-by-diagnosis interaction was observed (F=4.51, df=3, 54, p=0.04). While healthy youths and those with DBD were equally likely to accept fair offers and punished very unfair offers ($16/$4 and $18/$2) equally harshly, youths with DBD punished slightly unfair offers ($14/$6) more harshly than did healthy youths (
Table 1).
fMRI Results
The groups did not differ significantly in movement during scanning.
A three (diagnosis: DBD/+CU, DBD/–CU, healthy) by two (task phase: offer phase, decision phase) repeated-measures ANOVA was conducted on the BOLD data modulated by offer unfairness in the offer phase and punishment level in the decision phase (
Table 2; for complete results, see the supplemental results section and Tables S1–S3 in the online
data supplement).
Region-of-Interest Results
Amygdala.
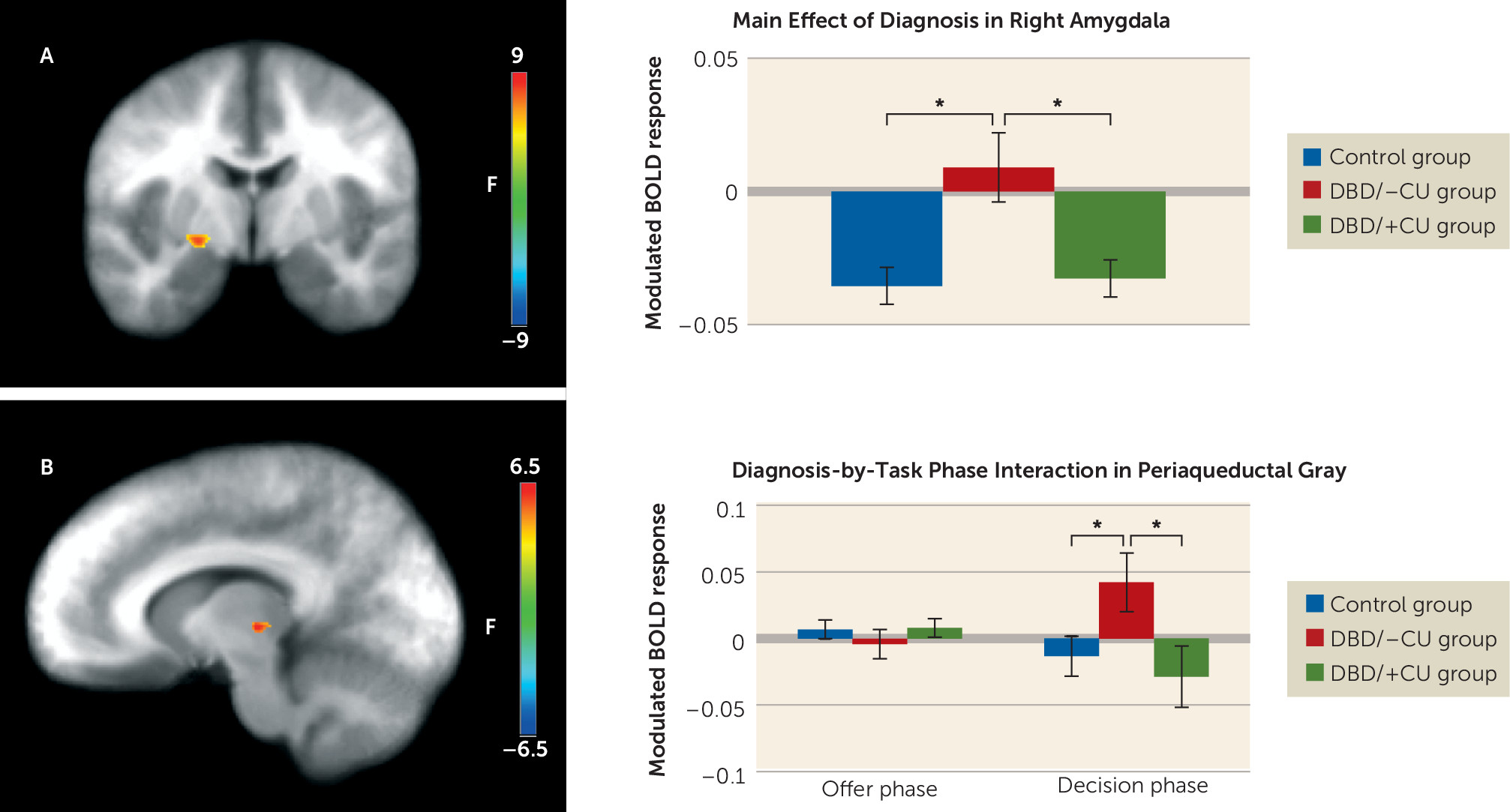
A significant main effect of diagnosis was observed (
Figure 2). Youths with DBD/–CU showed significantly greater right amygdala (coordinates: 23, −8, −6; k=10) responses relative to healthy youths (t=3.33, p<0.01) and youths with DBD/+CU (t=2.84, p<0.01), who did not differ significantly from each other. Among the youths with DBD, modulated amygdala response was inversely associated with callous-unemotional traits (r=−0.49, p<0.01], but not with reactive aggression.
Periaqueductal gray.
A significant diagnosis-by-task phase interaction was observed in the periaqueductal gray (coordinates: 10, −21, 4; k=6). During the decision phase, youths with DBD/–CU showed a significantly greater modulated periaqueductal gray activation relative to healthy youths (t=2.14, p=0.04) and youths with DBD/+CU (t=2.23, p=0.03), who did not differ significantly from each other. There were no significant between-group differences in the offer phase (
Figure 2).
Ventromedial prefrontal cortex.
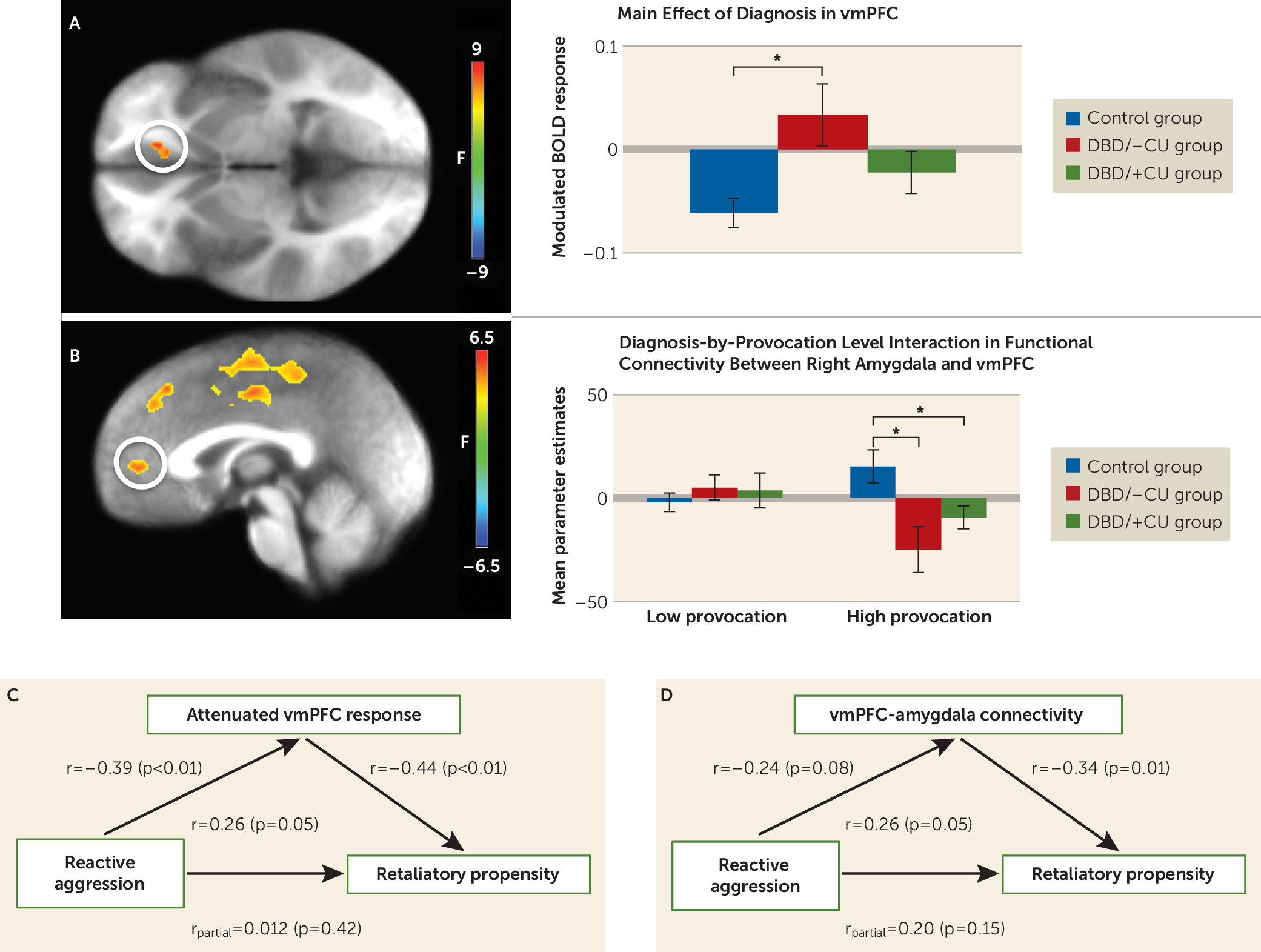
A significant main effect of diagnosis was observed, in which youths with DBD/–CU showed significantly reduced attenuation of modulated ventromedial prefrontal cortex (coordinates: −11, 33, −2; k=13) response relative to healthy youths (t=3.228, p=0.003). Youths with DBD/+CU did not differ significantly from youths with DBD/–CU or healthy youths (
Figure 3).
Generalized Psychophysiological Interaction Analyses
A significant diagnosis-by-provocation level interaction was observed in the ventromedial prefrontal cortex using the right amygdala seed (
Figure 3; see also the online
data supplement). During high provocation conditions, greater functional connectivity was observed between the right amygdala and the ventromedial prefrontal cortex in healthy youths relative to both youths with DBD/–CU (t=2.98, p<0.01) and those with DBD/+CU (t=2.16, p=0.04), who did not differ significantly from each other.
BOLD Response and Behavior
Retaliatory propensity was inversely associated with attenuation of modulated ventromedial prefrontal cortex response (r=−0.44, p<0.01) and ventromedial prefrontal cortex-amygdala connectivity during high provocation/retaliation conditions (r=−0.34, p=0.01). Given the significant relationship between retaliatory propensity and both modulated ventromedial prefrontal cortex response and ventromedial prefrontal cortex-amygdala functional connectivity, a hierarchical linear regression analysis was used to determine whether the modulated and connectivity data were independently related to retaliatory propensity beyond reactive aggression and callous-unemotional traits. Both modulated BOLD response (β=0.36, ΔR2=0.11, p<0.01) and functional connectivity in the ventromedial prefrontal cortex (β=−0.29, ΔR2=0.09, p=0.02) were observed to contribute unique variance in the prediction of retaliatory propensity.
Attenuation of modulated ventromedial prefrontal cortex response and retaliatory propensity were both significantly correlated with reactive aggression (r=−0.39 and r=0.26, respectively, p<0.05), although the correlation of ventromedial prefrontal cortex-amygdala connectivity with reactive aggression fell short of significance (r=−0.24, p=0.08). The relationship between retaliatory propensity and reactive aggression was not significant when controlling for attenuation in modulated ventromedial prefrontal cortex response or ventromedial prefrontal cortex-amygdala connectivity. However, the relationships between retaliatory propensity and attenuated ventromedial prefrontal cortex response (r
partial=−0.38, p<0.01) and ventromedial prefrontal cortex-amygdala connectivity (r
partial=−0.29, p=0.03) remained significant after controlling for reactive aggression and callous-unemotional traits. This suggests that modulated ventromedial prefrontal cortex response and ventromedial prefrontal cortex-amygdala connectivity mediate (
32) the association between reactive aggression and retaliatory propensity.
Confounding Factors
Among the youths with DBD, medication could not be withheld during scanning for 23.3% (N=7), and 50% (N=15) had comorbid ADHD. Therefore, the preceding analyses were rerun with these youths removed from the sample. In all regions, activations in similar regions were observed when only unmedicated youths were included and when youths with comorbid ADHD and DBD were excluded (see Tables S4–S7 in the online data supplement).
Discussion
To our knowledge, this is the first study to link retaliatory propensity to reactive aggression in youths with DBD. In addition, it provided the first demonstration of increased periaqueductal gray responding to provocation in youths with DBD/–CU, but not those with DBD/+CU. Critically, this study demonstrated the role of ventromedial prefrontal cortex dysfunction and ventromedial prefrontal cortex-amygdala functional connectivity in regulating retaliatory behavior. Furthermore, these results suggest that disruption in ventromedial prefrontal cortex-amygdala functional connectivity accounts for the common risk of increased retaliation/reactive aggression in DBD irrespective of callous-unemotional traits.
In line with our first prediction, retaliatory propensity on our ultimatum game task was significantly more positively associated with propensity for reactive relative to proactive aggression. Patients with DBD retaliated more severely during an ultimatum game, particularly to slightly unfair offers, than healthy youths. These data are consistent with other behavioral findings in antisocial adolescents (
8). In contrast, level of callous-unemotional traits did not predict level of reactive aggression (although it was associated with proactive aggression), consistent with previous research (
3).
According to our position (
15), while youths with DBD/+CU and DBD/–CU both show increased retaliatory behavior, the neurobiological underpinnings of the increase differ by level of callous-unemotional traits. Consistent with our second prediction, youths with DBD/–CU showed exaggerated basic threat circuitry (periaqueductal gray/amygdala) activation (
33) as a function of level of retaliation relative to youths with DBD/+CU and healthy youths. These data extend previous work reporting increased amygdala responses to provocation in youths with DBD/–CU relative to youths with DBD/+CU and healthy youths (
17–
19). According to our position, this heightened basic threat circuit sensitivity increases the likelihood of reactive aggression (rather than freezing or flight) in response to a threatening or provocative stimulus (
15). However, it should be noted that neither amygdala nor periaqueductal gray response predicted general propensity for retaliation in the task or reactive aggression levels more generally. Whether this reflects a type II error or is an indication that this pathology is more related to other associated symptomatology (e.g., anxiety) will be determined in future work.
Our third prediction was that youths with DBD, irrespective of level of callous-unemotional traits, are at increased risk for reactive aggression because of dysfunction in the ventromedial prefrontal cortex’s putative role in instrumentally selecting the form of retaliatory behavior as a function of expected rewards or punishments (
5,
14,
34). It is possible, however, that ventromedial prefrontal cortex responsiveness on this task might alternatively represent other putative roles for this structure—for example, direct suppression of the amygdala (
35), self-referential processing (
36), and representation of social and emotional structured event complexes (
37). We predicted that patients with DBD would show less reduction in ventromedial prefrontal cortex activity as a function of punishment level (i.e., less representation of the cost of retaliating) (
11). This hypothesis was confirmed for patients with DBD/–CU, but not those with DBD/+CU. However, patients in both DBD groups showed reduced functional connectivity between the right amygdala and the ventromedial prefrontal cortex during high provocation trials relative to healthy youths. This suggests a failure in the appropriate interaction of the amygdala and ventromedial prefrontal cortex in patients with DBD, irrespective of level of callous-unemotional traits, when responding to high provocation. In line with our fourth prediction, both modulated ventromedial prefrontal cortex response and ventromedial prefrontal cortex-amygdala functional connectivity contributed unique variance in the prediction of retaliatory propensity, and a mediation analysis supported the critical role of the ventromedial prefrontal cortex in regulating retaliation and reactive aggression. These data suggest that ventromedial prefrontal cortex-amygdala connectivity is critical for regulating retaliation and reactive aggression, describing putative common impairment in youths with DBD/+CU and DBD/–CU that contributes to retaliatory behavior.
Several caveats should be considered with respect to these data. First, an ADHD comparison group was not included, although a group analysis excluding youths with DBD and comorbid ADHD revealed similar results, with similar activations for all contrasts. Second, the medications of two youths with DBD could not be withheld for scanning; here again, group analyses excluding these participants identified similar regions for all contrasts. Third, the three-group analyses utilized relatively small samples. Thus, it is possible that type II error accounts for the failure of youths with DBD/+CU to show the hypothesized problems in BOLD response in the ventromedial prefrontal cortex as a function of punishment level (although this group did show the predicted reduced ventromedial prefrontal cortex-amygdala connectivity in response to high provocation). Our results in this study will need to be replicated with larger samples. Fourth, the periaqueductal gray is a small structure that is not expressly defined in AFNI’s probability maps. Therefore, we cannot be certain that the signal detected in the periaqueductal gray is exclusively from that structure; neighboring structures may also be involved.
In summary, three features of this study are particularly important for our understanding of disruptive disorders: First, this was the first study to document increased periaqueductal gray response to provocation in youths with DBD/–CU, but not in those with DBD/+CU. These data suggest that interventions designed to reduce emotional responsiveness would be most efficiently applied only to youths with DBD/–CU. Second, our results highlight the critical role of the ventromedial frontal cortex in the regulation of retaliatory behavior and that this structure is dysfunctional (at least its interaction with the amygdala) in patients with DBD irrespective of level of callous-unemotional traits. This dysfunction would appear to represent an important treatment target for future interventions. And third, level of ventromedial prefrontal cortex dysfunction in the ultimatum game task predicted level of reactive aggression in youths with DBD. This is important, as no fMRI markers of reactive aggression have been identified previously. Moreover, it is important to remember that the dysfunction seen here in patients with DBD is likely seen in patients with other psychiatric disorders who are at risk for maladaptively increased levels of reactive aggression, for example, posttraumatic stress disorder, borderline personality disorder, and disruptive mood dysregulation disorder (
38). Thus, this study provides a marker task of potential relevance to researchers who are concerned about maladaptively increased levels of reactive aggression both in patients with DBD and in those with other disorders.