Diminished attention to others’ eyes is a symptom of autism spectrum disorder (ASD) featured in each of the condition’s primary screening (
1) and diagnostic (
2) instruments. The behavior is among the earliest emerging features of the condition (
3), is well documented in individuals with ASD across the lifespan (
4), and contributes to deficits in initiating and modulating reciprocal social interaction (
5). Despite this clinical prominence, the underlying cause of atypical eye contact in ASD remains controversial.
Two explanations for reduced eye-looking in ASD are often advanced. One is that children with ASD purposefully look away from the eyes because of
gaze aversion—actively avoiding others’ eyes because the eyes are perceived as having negative affective valence (
6,
7). The other hypothesis is that children with ASD look less at others’ eyes because of
gaze indifference—looking less at others’ eyes because the eyes are not perceived as engaging or adaptively informative (
8,
9). Whereas gaze aversion indicates specific avoidance with implicit understanding of the social significance of eye contact (
10,
11), gaze indifference indicates insensitivity to the underlying social signal of others’ eyes, part of a broader insensitivity to social cues in general (
12). At a mechanistic level, these hypotheses are mutually exclusive: one posits responses based on distinct and differential awareness of the eyes, the other posits responses based on the relative absence of that awareness.
The evidence for and against each hypothesis is mixed. In support of gaze aversion, studies of affective response to gaze have reported increased autonomic arousal (
13,
14), amygdala activation (
15,
16), and self-report of threat and anxiety associated with eye-looking in older children and adults with ASD (
11,
17). Within this model, avoiding others’ eyes represents a motivated response to withdraw in order to prevent negatively valenced, overstimulating hyperarousal (
13,
18). Hyperactivation of the amygdala and of the sympathetic system is thus considered the cause rather than a consequence of atypical eye-looking in ASD.
In contrast, in support of gaze indifference, many areas of the “social brain” system in individuals with ASD, including the amygdala (
19), are hypoactive to social stimuli in general (
20) and hyporesponsive to gaze cues in particular (
21). The gaze indifference hypothesis holds that children with ASD, unlike typically developing children (
22), are insensitive to underlying social signals from another person and therefore do not perceive others’ eyes as adaptively informative or socially salient, leading to passive omission of eye-looking (
23). Insensitivity to the social salience of the eyes atypical developmental specialization of the “social brain” network, including the right posterior superior temporal sulcus, which is typically attuned to the inferred communicative intentionality of eye gaze (
24), and the basolateral nuclei of the amygdala, which typically contribute to attribution of reward value and to processing the relative salience of stimuli (
25,
26).
Distinguishing between these two hypotheses has critical implications both for treatment and for our mechanistic understanding of the condition. If gaze aversion underlies diminished eye contact in ASD, anxiolytics (e.g.,
26) or behavioral interventions that increase exposure to others’ eyes (e.g.,
27) may be indicated to address social impairments. Alternatively, if gaze indifference underlies diminished eye contact in ASD, interventions aimed at enhancing social engagement and the reinforcer value of social interaction (e.g.,
28) may be indicated.
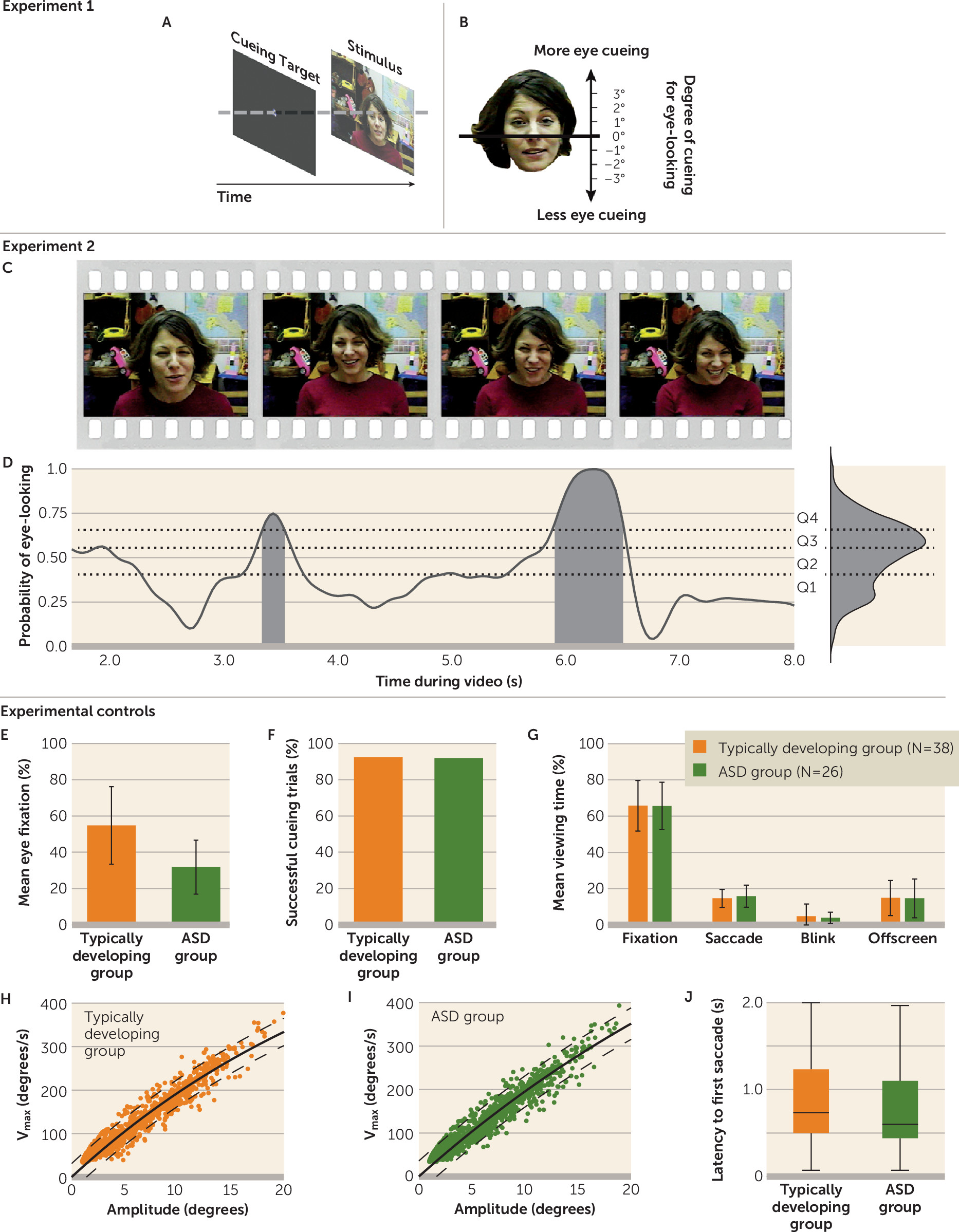
To directly test these two hypotheses, we collected eye-tracking data at the time of initial diagnosis in both 2-year-olds with ASD and matched children. In experiment 1, children were directly cued to look either more or less at the eyes by presenting a target stimulus directing gaze to specific locations (
Figure 1A,B). In experiment 2, rather than directly cueing a child for eye-looking, we measured levels of eye-looking during periods of time-varying implicit cueing for eye-looking (
Figure 1C,D).
Method
Participants
Eighty-six children (26 with ASD, 38 typically developing, and 22 non-ASD developmentally delayed) with a mean age of 24.9 months (SD=7.5) participated. Children with ASD were consecutive community referrals to a diagnostic clinic. Experimental data were collected at the time of initial diagnosis. All children met criteria for autistic disorder or ASD on the Autism Diagnostic Observation Schedule, Second Edition, module 1 (
2); met criteria for autistic disorder or ASD on the Autism Diagnostic Interview–Revised (
29); and received a diagnosis of autistic disorder (19 of 26 children) or pervasive developmental disorder not otherwise specified (seven of 26 children) by two experienced clinicians. At the time of assessment, diagnostic guidelines followed DSM-IV-TR criteria; all children in the ASD group also met DSM-5 criteria for ASD. Of the 26 toddlers with ASD, 20 were seen again after age 3, and all still met diagnostic criteria for ASD. Children in the typically developing group all exhibited no developmental delays (measured as any single delay of more than two standard deviations or as two delays each greater than 1.5 standard deviations on the visual reception, receptive, or expressive language subscales of the Mullen Scales of Early Learning [
30]), had no known genetic syndrome, and had no family history of ASD in first- or second-degree relatives. Children in the developmentally delayed group did not meet diagnostic criteria for ASD and exhibited single delays of more than two standard deviations or two delays of 1.5 standard deviations on the visual reception, receptive, or expressive language subscales of the Mullen Scales of Early Learning. None of the participants were receiving intervention services at testing, which was the time of initial diagnosis for all participants with ASD and non-ASD developmental delays.
Toddlers with ASD were matched on sex, chronological age, and nonverbal cognitive ability to typically developing toddlers and were matched on sex, chronological age, and verbal cognitive ability to the developmentally delayed group (see Table S1 in the data supplement that accompanies the online edition of this article).
Parents or legal guardians provided written informed consent for participation. The Institutional Review Board at Yale University approved the research protocol. All aspects of the experimental protocol were performed by personnel blind to diagnostic status of the children. Diagnostic measures were administered by trained clinicians blind to the results of the experimental procedures.
In both experiments, eye-tracking data were collected by a dark pupil/corneal reflection technique (using equipment from ISCAN, Inc., Woburn, Mass.) while children viewed video scenes of an actress looking directly into the camera, portraying the role of a caregiver and speaking to the viewer in toddler-directed speech. Actresses were filmed in front a background that approximated a child’s room, including colorful pictures and shelves of toys. For a detailed description of stimuli and data collection and processing, please see the online data supplement.
Experiment 1: Response to Direct Cueing for Eye-Looking
In the first experiment, children were directly cued to look at the eyes, the mouth, or nonface regions by a prestimulus cueing target (see
Figure 1A). The cueing target was standardized at the center of the stimuli presentation monitor, and the location of face stimuli varied with respect to the cueing target location across trials. Trials in which an individual did not look at the cueing target location were excluded from analysis. For the dependent variables, we measured, for each child, latency to look away and sustained levels of continued eye-looking after cueing and after initial shifts of gaze.
Cueing effects on latency to first saccade.
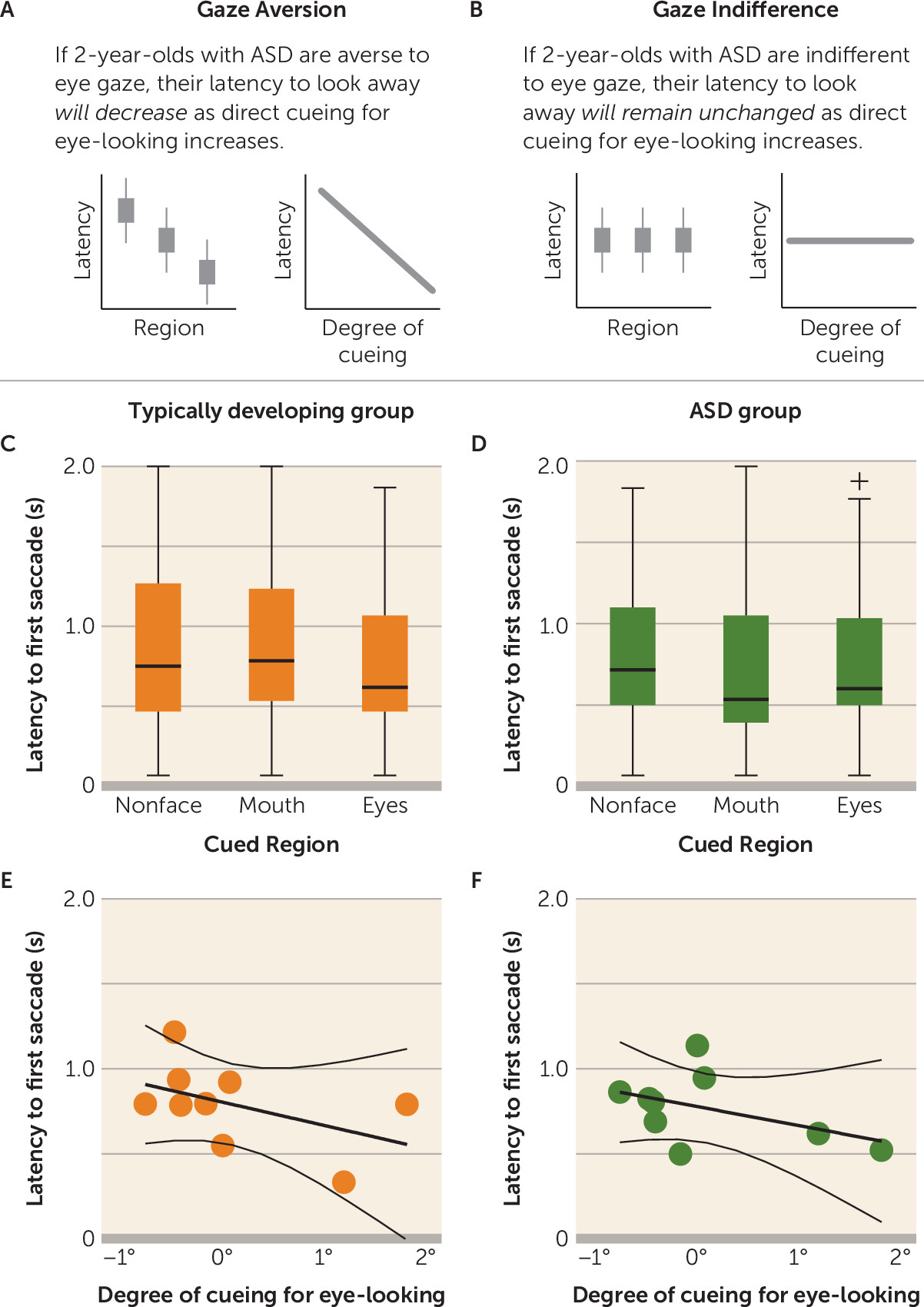
For latency to look away, the gaze aversion hypothesis predicted a significant negative association: if children with ASD were averse to eye-looking, latency to first saccade would decrease (looking away more quickly) as direct cueing for eye-looking increased (
Figure 2A). In contrast, gaze indifference predicted no relationship: if children with ASD were indifferent to eye-looking, latency to look away would remain unchanged as direct cueing for eye-looking increased (
Figure 2B).
Comparisons were made both categorically (cued to eyes, mouth, or nonface regions) and continuously (closer to or farther from the eyes, measured in degrees of visual angle; see
Figure 1B). Categorical hypotheses were tested by within-group one-way analyses of variance (ANOVAs) evaluating latency to look away as a function of cueing target region (eyes, mouth, and nonface regions). Dimensional hypotheses were tested by Pearson correlation coefficient to measure the association between latency and degree of cueing.
Postcueing effects on sustained levels of eye-looking.
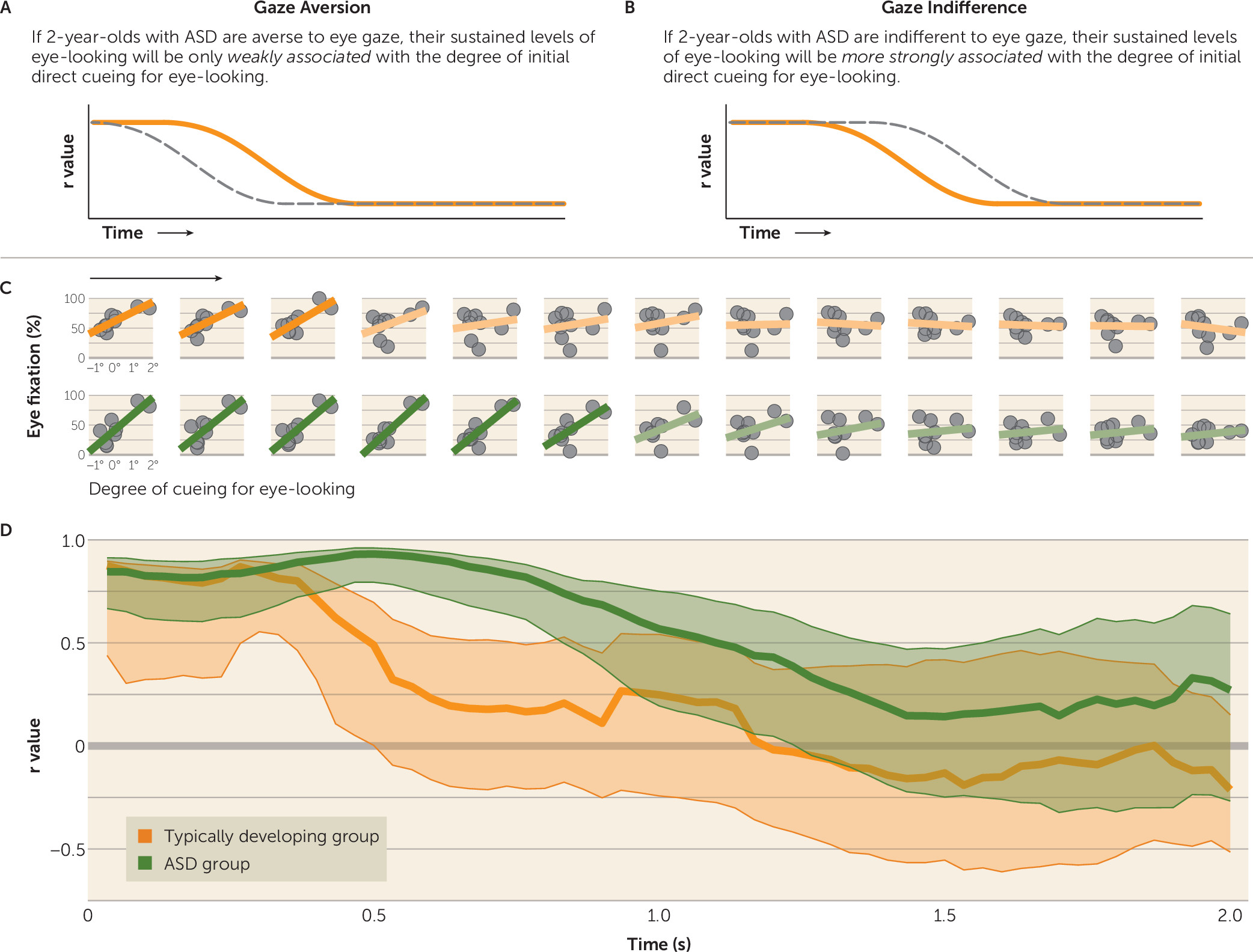
Participants’ initial fixation location was determined by cueing target location, but subsequent fixation locations and sustained levels of eye-looking were determined by the participants themselves, freely shifting their gaze. The gaze aversion hypothesis predicted a weaker association in the ASD group between initial degree of direct cueing and subsequent levels of eye-looking: after being cued to look at the eyes, children with ASD, even if not initially faster to look away, would still avoid the eyes over time, using subsequent saccades to look away (
Figure 3A). In contrast, gaze indifference predicted a stronger association in the ASD group between initial degree of direct cueing and subsequent levels of eye-looking: after being cued for eye-looking, children with ASD would continue to look more at the eyes (
Figure 3B).
We tested these hypotheses by measuring the association, at each moment in time, between degree of initial direct cueing for eye-looking and subsequent percentage of actual eye-looking. Associations were measured by Pearson correlation coefficient, with between-group analyses conducted by comparison of bootstrapped means and 95% confidence intervals calculated across 5,000 resamplings.
Experiment 2: Response to Implicit Cueing for Eye-Looking
In the second experiment, we measured levels of eye-looking during periods of time-varying implicit cueing for eye-looking.
Implicit cueing for eye-looking.
Given previous research in typically developing children demonstrating intrinsic engagement and responsiveness to the underlying social cueing of the eyes (
31,
32), we quantified the strength of implicit cueing for eye-looking in our video stimuli on the basis of data from typically developing children. Specifically, after the period in which direct cueing was no longer associated with fixation location (t>1,500 ms), we measured the time-varying probability that typically developing toddlers would look at the eyes (
Figure 1C). This moment-by-moment variation in probability of eye-looking reflects, for typically developing toddlers, their normative response to cues within the video (i.e., naturally occurring social cues proffered by each actress while speaking to the viewer in toddler-directed speech). The probability of eye-looking varied between 0 and 1 and was measured at 33.3-ms intervals. This probability, ranked by quartiles, served as our index of implicit cueing (
Figure 1D). To test the validity of these cues, we used leave-one-out cross-validation (
33). A within-group repeated-measures ANOVA across all leave-one-out cross-validation comparisons showed high predictive validity for increased eye-looking (F=34.30, df=3, 111, p<0.001).
For the dependent variables, in relation to strength of implicit cueing, we then measured the probability of eye-looking and the density of fixation locations for each child.
Implicit cueing effects on probability of eye-looking.
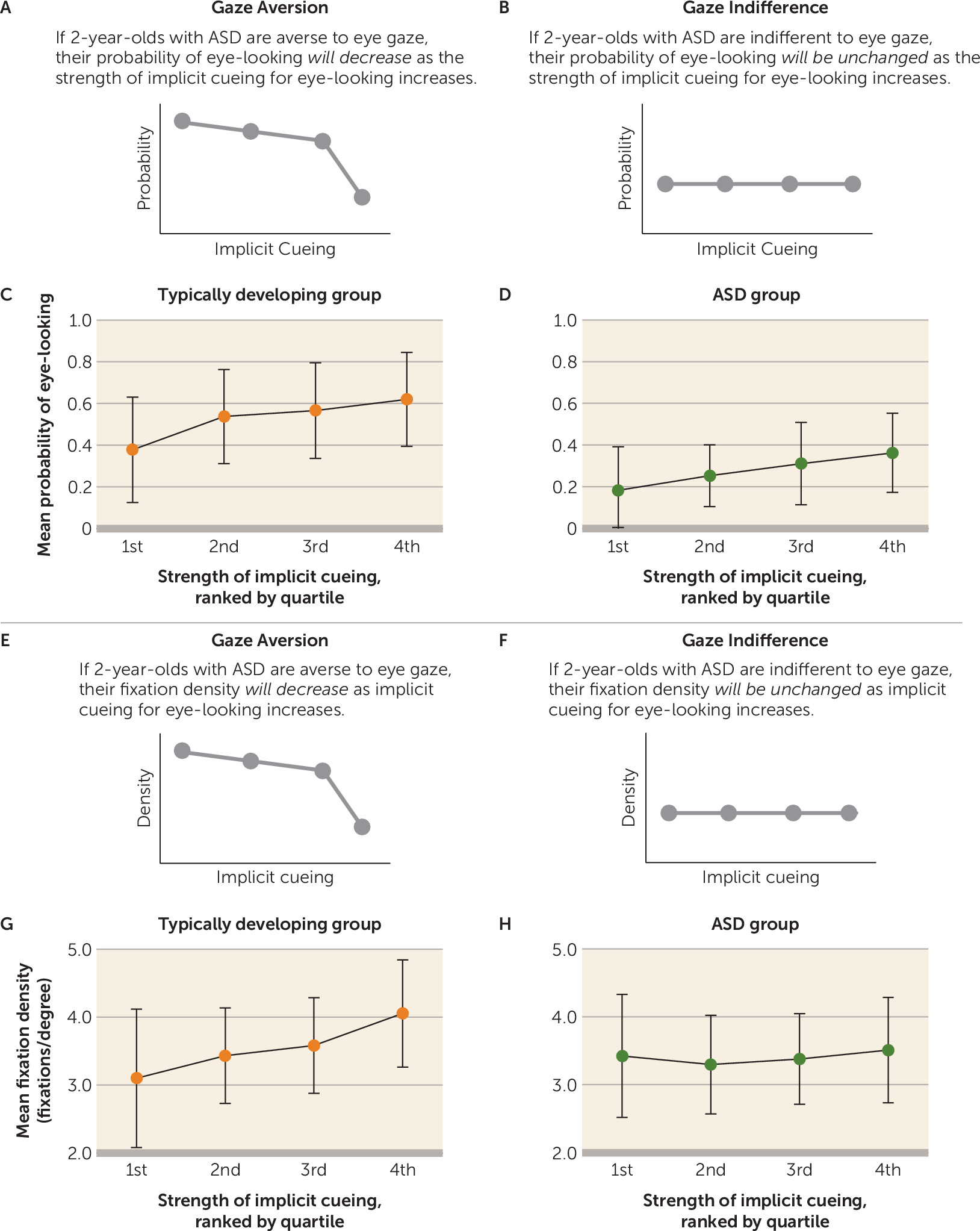
The gaze aversion hypothesis predicted a decline in probability of eye-looking as the strength of implicit cueing increased: children with ASD would avoid the eyes specifically at moments with strongest implicit cueing for increased eye-looking (
Figure 4A). In contrast, gaze indifference predicted no change in the probability of eye-looking as a function of implicit cueing; children with ASD would be insensitive to implicit cues (
Figure 4B). Effects of implicit cueing on probability of eye-looking were measured by within-group repeated-measures ANOVA.
Implicit cueing effects on fixation density.
Rather than fully shifting gaze away from the eyes, it was also possible that children with ASD would more subtly reorient gaze toward peripheral locations when implicitly cued for eye-looking. The gaze aversion hypothesis predicted a decline in viewers’ fixation density relative to level of implicit cueing: at times of high implicit cueing, children with ASD would shift gaze to the periphery (
34), resulting in more widely distributed fixations (
Figure 4E). Alternatively, the gaze indifference hypothesis predicted no relationship: children with ASD would not reorient their gaze specifically in response to implicit cues, resulting in unchanged fixation density (
Figure 4F). Fixation density was measured by kernel density analysis (
35) at 33.3-ms intervals. The effect of implicit cueing for increased eye-looking on fixation density was assessed using within-group repeated-measures ANOVAs.
Results
Throughout free-viewing of the video scenes, we replicated a reduction in eye-looking in the ASD group compared with typically developing and developmentally delayed children (
36) (
Figure 1E; see also Table S1 in the
online data supplement), indicating diminished visual attention to others’ eyes, the basic behavioral criterion for either gaze aversion or indifference. Across all analyses, results in the developmentally delayed group were comparable to those of the typically developing group, providing evidence that differences observed in the ASD group were due to diagnostic status rather than to verbal functioning. For a detailed description of results in the developmentally delayed group, please refer to the
online data supplement.
Response to Direct Cueing for Eye-Looking
As experimental controls, we tested whether the ASD and typically developing groups differed in successful direct cueing (typically developing group, mean=92%; ASD group, mean=92%; χ
2=0, p>0.999) (
Figure 1F), time spent freely watching the stimuli (typically developing group, mean=65.8%, SD=13.9; ASD group, mean=65.6%, SD=13.1; t=0.05, df=1, 62, p=0.96; d=0.01) (
Figure 1G), and integrity of extraocular muscle movements (for control of saccade amplitude and velocity;
Figure 1H,I). In all cases, there were no significant between-group differences.
Given previous studies of reaction time to disengage in children with ASD (
37), reaction time following direct cueing was measured across all cueing trials. Latency to first saccade was not significantly different between groups (typically developing group, mean=0.86 seconds, SD=0.52; ASD group, mean=0.76 seconds, SD=0.48; t=1.42, df=1, 361, p=0.16, d=0.15) (
Figure 1J), indicating that the typically developing and ASD groups were equally capable of rapidly shifting gaze.
We then measured latency to first saccade as a function of cueing target location. There was no significant relationship between latency to first saccade and degree of eye cueing in the typically developing (
Figure 2C,E) or the ASD group (
Figure 2D,F), measured either categorically (
Figure 2C,D) (typically developing group: F=1.13, df=2, 197, p=0.33; η
p2=0.01; ASD group: F=0.65, df=2, 160, p=0.52; η
p2=0.01) or continuously (
Figure 2E,F) (typically developing group: r=−0.40, p=0.28; ASD group: r=−0.39, p=0.30).
Next, we examined the effects of direct cueing for eye-looking on sustained (postcueing) levels of eye-looking. At trial onset, level of eye-looking was significantly associated with degree of eye cueing in both the typically developing (r=0.88, p=0.002) (
Figure 3C) and ASD groups (r=0.85, p=0.004), indicating that participants in both groups had been successfully cued by the prestimulus target. For between-group analyses, we compared bootstrapped 95% confidence intervals of the r value of the correlation over time. At trial onset, as expected, bootstrapped confidence intervals for each group overlapped, but beginning at 470 ms after cueing, the association between degree of initial cueing and level of eye-looking was significantly greater in the ASD group than in the typically developing group (
Figure 3D). The significant association between eye cueing and level of eye-looking persisted in the ASD group until 1,233 ms, a more than twofold increase in duration of the effect of direct cueing in the ASD group compared with the typically developing group (for whom the effect lasted 500 ms).
The observation of a stronger association between eye-looking and initial direct cueing in the ASD group (
Figure 3D) occurred despite equivalent latencies to first saccade. Stated differently, the persistence of this association required both additional fixations on the eyes when cued for eye-looking as well as additional fixations on the mouth when cued for mouth-looking, an indication of relative insensitivity to the content of either cued target location.
Response to Implicit Cuing for Eye-Looking
We measured probability of eye-looking as a function of implicit cueing for eye-looking. In the typically developing group, by leave-one-out cross-validation, we observed a significant main effect of strength of implicit cueing (F=34.30, df=3, 111, p<0.001; η
p2=0.48) (
Figure 4C). There was also a significant main effect in the ASD group (F=12.78, df=3, 75, p<0.001; η
p2=0.34) (
Figure 4D). In contrast to our predictions based on the gaze aversion and indifference accounts, children with ASD were more likely to look at the eyes at times with stronger implicit cueing. Critically, however, children with ASD were not less likely to look at the eyes in response to the strongest cueing for eye-looking, evidence that is inconsistent with the gaze aversion hypothesis.
Next, we examined fixation density as a function of implicit cueing. In the typically developing group, there was a significant main effect of implicit cueing (F=23.69, df=3, 111, p<0.001; η
p2=0.39) (
Figure 4G). In contrast, in the ASD group, there was no change in fixation density based on strength of implicit cueing (F=1.60, df=3, 75, p=0.20; η
p2=0.06) (
Figure 4H).
Discussion
We studied two mechanistic hypotheses for diminished eye-looking in ASD: gaze aversion and gaze indifference. The distinction between these hypotheses is critical because the two posit different causal mechanisms and different target areas of atypical brain function. Ultimately, the two hypotheses present different conceptions of ASD and indicate different approaches to behavioral and pharmacological treatment.
Across all analyses, the results were inconsistent with the gaze aversion hypothesis. At the time of initial diagnosis, 2-year-olds with ASD did generally look less at the eyes, but they looked less at the eyes in a fashion that could be overridden by direct cueing for eye-looking and in a fashion that was not systematically or consistently driven by implicit social cueing. When directly cued to look at the eyes (experiment 1), 2-year-olds with ASD did not look away faster than typically developing children. The effects of direct cueing were actually stronger in 2-year-olds with ASD than in typically developing children (when cued to look at the eyes, they looked longer at the eyes; when cued to look at the mouth, they looked longer at the mouth). Moreover, when presented with implicit social cues for eye-looking (experiment 2), there was no evidence of 2-year-olds with ASD shifting their gaze away from the eyes at specific moments of high implicit eye salience.
These results contradict the hypothesis that children with ASD actively avoid looking at the eyes in early life. Instead, the results are consistent with gaze indifference and indicate passive insensitivity to social signals in others’ eyes coupled with intact sensitivity to nonsocial, physical cues (
38). Our results are also consistent with existing evidence on response to directional eye gaze cues in toddlers with ASD. Similar to typically developing toddlers, toddlers with ASD orient their attention based on eye gaze cues in structured experimental situations (i.e., Posner-like cueing tasks) (
39). However, unlike typically developing toddlers, toddlers with ASD do not show a differential response or distinct sensitivity to eye gaze cues compared with nonsocial cues (e.g., arrows) (
32). Even when toddlers with ASD exhibit superficially typical responses to gaze cues in experimental settings, they do not demonstrate spontaneous response to gaze cues (i.e., response to joint attention) in less structured interactions (
32). Similarly, even at older ages when children with ASD more consistently demonstrate response to joint attention, which is associated with developmental level (
39), they show consistent impairments in initiating joint attention with others (
40) and in inferring others’ mental states from gaze cues (
41), providing additional evidence of gaze indifference.
In finding that the results of these experiments are inconsistent with and cannot support the gaze aversion hypothesis, we do not mean to suggest that all other evidence supportive of gaze aversion is invalid. Previous findings of atypical autonomic response (
13,
14), atypical amygdala activation (
15,
16), and increased anxiety (
11,
17) in response to gaze have focused on older children and adults with ASD. Similarly, clinical observations (
6) and first-person reports (
42) of anxiety or aversion in response to others’ eyes are generally from later childhood or adulthood. The age contrast between the present study and later-life accounts is an important source of information. In older children and adults, atypical affective response to eye gaze may be a developmental consequence rather than cause of atypical eye-looking. Consistent with this, the prevalence of anxiety symptoms in ASD is positively associated with age (
43) and is moderated by cognitive functioning (
44), suggesting that anxiety symptoms are late emerging and do not underlie core social deficits in ASD. Additionally, EEG frontal asymmetry, which provides evidence of approach-withdrawal responses (
45), suggests that hyperactivation and hyperarousal to the eyes in older individuals with ASD may not be negatively valenced (
23,
46), arguing against the interpretation of atypical autonomic and amygdala activity as indicating aversion.
If at the time of initial diagnosis toddlers with ASD passively omit eye contact and do not show clear signs of actively avoiding the eyes of others (as in the present experiments), and if, at a later time point, some individuals with ASD either exhibit or self-report anxiety-related reactions to eye-looking (which result in their actively avoiding the eyes of others), what changes transpired between the early and later time points? The answer to that question may help shed light on learning processes underlying the natural history of secondary symptomatology in ASD, that is, symptoms that emerge as a result of compensatory processes rather than mechanisms that have a causative role in pathogenesis (please see the online data supplement for an extended discussion).
These findings provide important points for future study of the neuropathology of ASD. Our results indicate that reduced eye-looking in ASD at the time of initial diagnosis is not an anxiety-related response and that it is unlikely to be caused by hyperarousal or amygdala hyperactivation. Instead, because reduced attention to the eyes appears to be due to passive insensitivity to the social signals of a conspecific, observed amygdala dysfunction in ASD is more likely due to atypical development of neural networks involving the basolateral amygdala, including circuits associated with social gaze perception and with frontal cortex-associated attribution of reward value to social interaction (
25,
26,
47). Thus, rather than focusing on emotion-regulation and threat-processing pathways, future studies may focus more productively on developmental specialization of social brain networks that subserve engagement with and valuation of socially relevant stimuli in hopes of better understanding and ultimately treating the neural mechanisms of gaze indifference as well as associated, and more general, impairments in social adaptive action in ASD.
Acknowledgments
The authors gratefully acknowledge the children and families for their participation. They thank Laura Edwards, Sarah Shultz, Katherine Rice, Jessie Northrup, and Gordon Ramsay for discussions of data analysis; Casey Zampella and Tawny Tsang for assistance with measures of extraocular muscle movements; and Nathan Call and Chris Gunter for assistance in reviewing drafts of the manuscript.