Attention deficit hyperactivity disorder (ADHD), pediatric obsessive-compulsive disorder (OCD), and autism spectrum disorder (ASD) are relatively common childhood neurodevelopmental disorders (NDDs). In line with evidence that the higher-order tasks that are impaired in NDDs (e.g., social communication in ASD, regulation of attention and behavior in ADHD and OCD) rely on tight control of activation across neural networks (
1), neuroimaging studies have recently been focused on understanding network connectivity in NDDs. Although published studies consistently implicate differences in structural and functional connectivity measures in a single NDD group, compared with controls (
2–
4), we have yet to uncover disease-specific neurobiological features for any NDD. Consistent with neuroimaging findings that are not specific to any one NDD, considerable phenotypic (
5,
6) and genetic overlap has been found between NDDs (
7). For example, some of the same genes that are involved in regulating neural migration and synaptic development (e.g., ASTN2, contactin-associated proteins) have been implicated across cases of OCD, ASD, and ADHD (
7,
8). Nonspecific and overlapping findings across these disorders suggest that early developmental disruption of neural connections may be a common etiopathogenic risk factor across different NDDs.
Diffusion tensor imaging (DTI) is the only MRI-based neuroimaging method that can infer properties of structural brain connectivity in vivo. Fractional anisotropy, the most widely reported DTI index in NDDs (
2,
4,
9,
10), provides a measure of directionally dependent water molecule diffusion that correlates with coherence and integrity of neural fibers (
11). Voxel-wise analyses applied to DTI data enable exploration of white matter microstructure across the brain (large-scale structural connectivity). In NDDs, voxel-wise DTI studies largely point to decreased fractional anisotropy affecting widespread regions housing white matter connections in ASD (
2), increased and decreased fractional anisotropy most commonly localized to the anterior corona radiata, internal capsule, corpus callosum, and cerebellar white matter in ADHD (
4), and fractional anisotropy values that are either no different or higher for voxels along the cingulum bundle and corpus callosum in OCD (
3), compared with controls. However, small sample sizes, and conflicting results, continue to limit our understanding of how neural connectivity differs in NDDs.
To date, no voxel-wise DTI study, to our knowledge, has directly compared different NDDs with each other (
12,
13). Direct comparison of different NDDs may provide new understanding of biological mechanisms that are either shared between disorders or unique to any one disorder. These types of insight may be vital for treatment innovation and development of biologically informed classification systems. In addition, some symptoms and general functional impairment are often shared across NDDs (e.g., attention problems in ADHD and ASD [
6], repetitive behaviors in ASD and OCD [
5], general functional impairment across ADHD, ASD, and OCD [
14,
15]). Exploration of the relations between neural circuitry and dimensional behavioral impairment may uncover brain-behavior relationships in a manner not possible using a categorical, disease-based approach (
16).
In the present study, Tract-Based Spatial Statistics (
17), a voxel-wise approach optimized for examination of diffusion properties of white matter across the brain, was applied in a relatively large sample including children and adolescents diagnosed with ADHD, ASD, or OCD and controls. Our primary aims were to 1) compare DTI indices across NDD groups and controls to see whether the direction and extent of white matter alterations were distinct for different NDDs and 2) when “lumping” NDD participants together, to assess differences with controls and relations with clinical symptom dimensions that cut across NDDs. Based on previous case-control DTI studies, we hypothesized that 1) fractional anisotropy would be lower in corpus callosum and fronto-striatal (corona radiata and internal capsule) white matter in ASD and ADHD but not OCD (
2–
4), when compared with controls, respectively, and 2) fractional anisotropy would correlate with disease burden for clinical symptoms that cut across NDDs.
Discussion
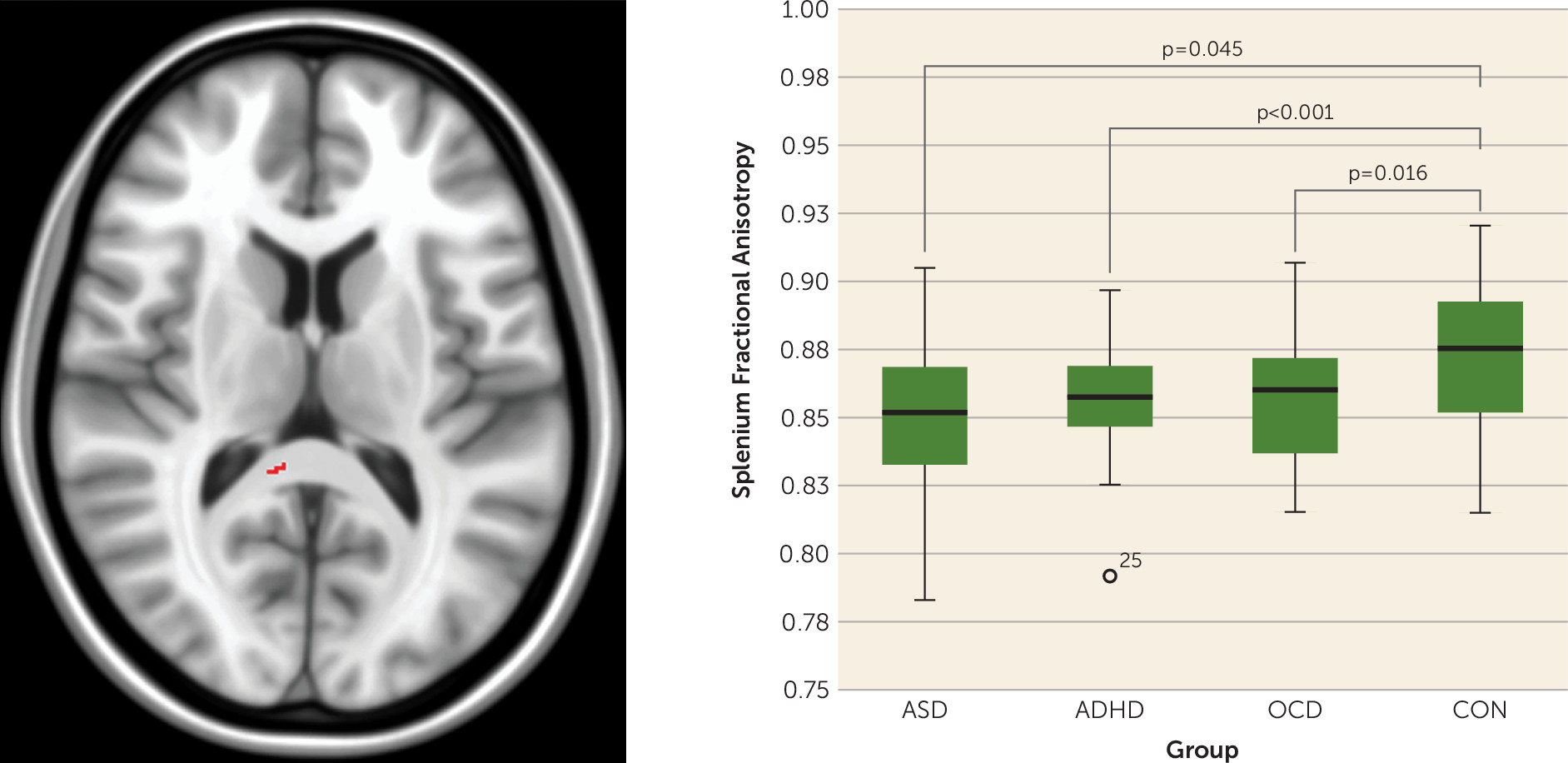
In the present study, we examined distinct and nondistinct features of structural brain connectivity in a large sample that included children and adolescents with and without NDDs. First, we found lower fractional anisotropy in voxels along the splenium of the corpus callosum in all three NDD groups, compared with the control group. Pairwise comparisons revealed that both the ASD and ADHD groups had reductions in fractional anisotropy in a number of additional white matter tracts, compared with controls, that were not found in the OCD group. No significant fractional anisotropy differences were found between the ASD and ADHD groups; however, reduced fractional anisotropy was present in both the ASD and ADHD groups compared with the OCD group. Examination of NDD participants together, compared with controls, revealed lower fractional anisotropy in NDD individuals in voxels along the genu and splenium of the corpus callosum, cortico-spinal tract, fronto-occipital, fronto-temporal, and occipito-temporal white matter connections; fractional anisotropy within these same white matter tracts was also associated with adaptive functioning across all NDD participants.
Our finding of reduced fractional anisotropy in the splenium that was not different between NDD groups is consistent with prior pediatric case-control studies finding lower splenium fractional anisotropy in either ADHD or ASD samples, compared with controls (
2,
4). Splenium fractional anisotropy differences have also been shown in OCD samples compared with controls, although the direction of findings varies (
3). Lower fractional anisotropy in brain white matter is reflective of decreased directional organization of neural fibers and may be due to changes in axon diameter, density, axonal packing properties, or myelin integrity (
11). The corpus callosum facilitates interhemispheric connectivity/inhibition across the cortex (
31). Corpus callosum structure in infancy predicts executive function in childhood and has been shown to correlate with tasks related to cognition in preschool-age children (
32). Corpus callosum axons undergo extensive refinement in the postnatal period, and splenium fractional anisotropy increases rapidly over the first 10 years of life (
33), a period that overlaps with the onset of NDDs. Given the role of the corpus callosum in interhemispheric connectivity across the cortex, early developmental disruption within this tract may influence structural development in related long-range white matter tracts that undergo more protracted maturation. In addition to fractional anisotropy findings, we found lower axial (but no difference in radial or mean) diffusivity in NDDs compared with controls in the present study (see the
online data supplement). Although previous studies have interpreted axial and radial diffusivity differences in NDDs as a specific indicator of axonal or myelin disruption, respectively (
2–
4), evidence has indicated that this measure is sensitive to a number of additional factors in humans and may not be an appropriate indicator of specific tissue pathology (
34).
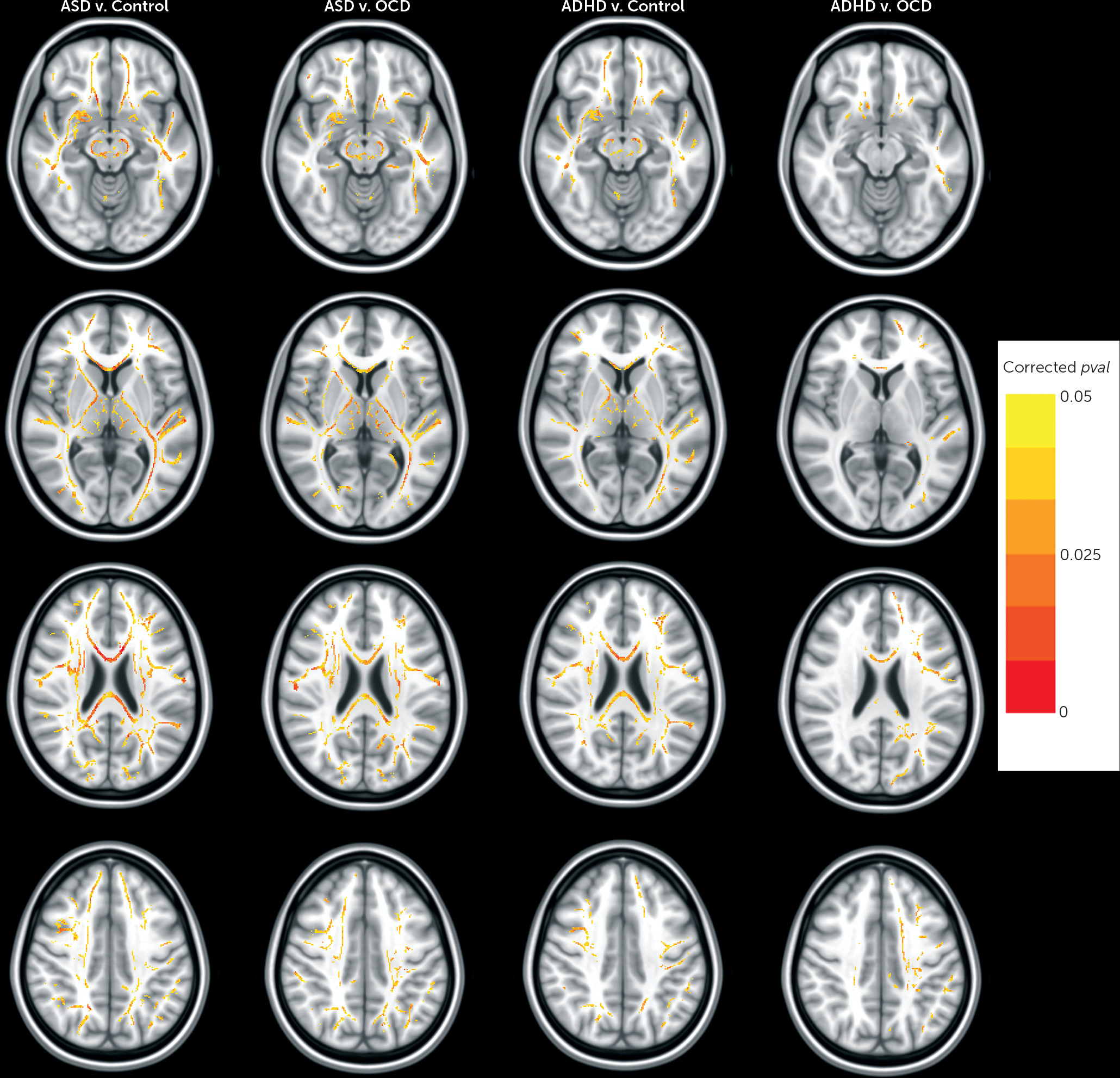
In line with several previous studies, case-control comparisons showed lower fractional anisotropy that was neuroanatomically widespread in ADHD or ASD, compared with controls (
4). However, no fractional anisotropy differences were found in the ADHD group compared with the ASD group, and fractional anisotropy differences did not extend beyond the splenium in the OCD group compared with the control group. Our results in ADHD and ASD are consistent with two recent imaging studies that directly compared connectivity patterns in children and adolescents with ASD or ADHD and controls (
35,
36). These studies indicated that both ASD and ADHD are disorders involving large-scale connectivity impairments based on the diffuse nature of structural and functional connectivity differences found across the brain in each NDD compared with controls. The presence of a similar scale of disrupted connectivity in ASD and ADHD is consistent with the early timing of onset for each disorder, which corresponds to a period of dynamic maturational change for long-range white matter tracts across the brain (
33). It is also consistent with evidence of enrichment for rare genetic variants that encode cell-adhesion and neuronal synapse scaffolding in both conditions (
7). Although we did not find disease-specific fractional anisotropy alterations in ASD or ADHD in the present sample, two previous studies found differences between these disorders in terms of network topology (
35,
36).
In contrast to the absence of significant differences between ADHD and ASD, we found differences in white matter structure in both the ASD group and the ADHD group, compared with the OCD group. Lower fractional anisotropy in ASD compared with OCD was widespread, while fractional anisotropy reductions in ADHD compared with OCD included voxels along the cortico-spinal tract, genu of the corpus callosum, and inferior-fronto-occipital fasciculus, frontal white matter connections that have been implicated in both ADHD and OCD previously (
13,
37). It may be that the later timing of disease onset in pediatric OCD (middle-childhood as opposed to early childhood in ASD and ADHD) explains the more substantial and widespread fractional anisotropy reductions found in children and adolescents with ASD and ADHD compared with OCD in our sample. Post hoc analyses indicated that fractional anisotropy differences in fronto-cortical white matter tracts in the ADHD group compared with the OCD group were also negatively correlated with attention problems (characteristic ADHD symptoms) and positively correlated with obsessive-compulsive symptoms in participants with either ADHD or OCD (see the
data supplement). Positive correlations between fronto-cortical fractional anisotropy and OCD symptoms (
3) and negative associations between fronto-cortical fractional anisotropy and ADHD symptoms (
4) have been found in separate case-control studies examining OCD and ADHD groups, compared with controls, respectively. These converging results may shed light on how divergent structural properties within the same fronto-cortical network may influence expression of seemingly opposing clinical symptoms (inattentive/impulsive versus obsessional/compulsive symptoms). However, these results must be considered preliminary, given the sample size of our pairwise ADHD-OCD comparison and the presence of diagnostic overlap in a small number of included participants.
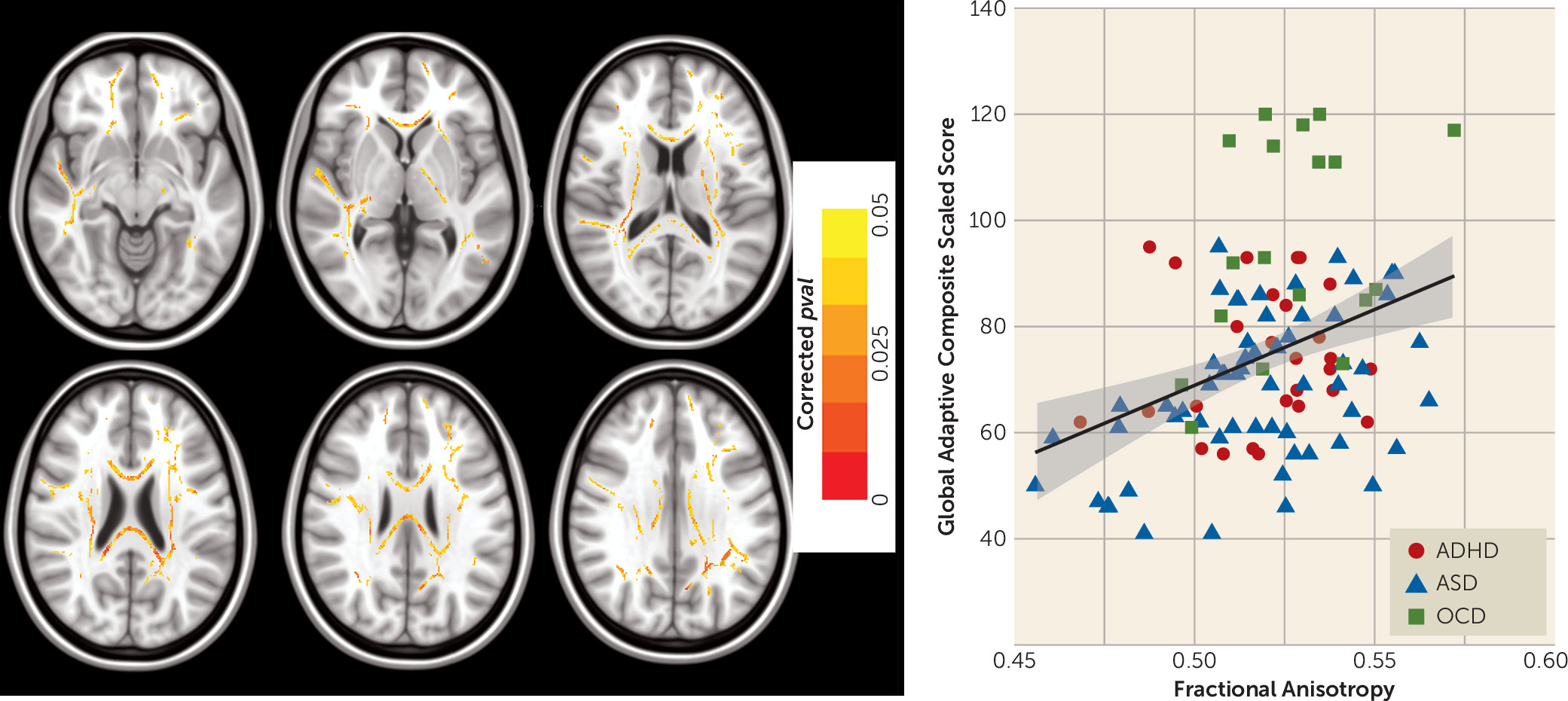
Finally, we found lower fractional anisotropy in individuals with an NDD compared with controls in voxels along a number of long-range white matter connections with particularly prominent effects on the genu and splenium of the corpus callosum. Our brain-behavior analyses across all NDDs showed that general adaptive functioning was positively correlated with fractional anisotropy in voxels along major interhemispheric and cortico-cortical connections. The tissue properties of long-range white matter connections are thought to facilitate control over the speed and timing of activation across neural networks and are of critical importance for efficient performance in higher-order tasks (
1) that support adaptive functioning. Impaired adaptive functioning is a part of the diagnostic criteria for each of the NDDs sampled in the present study (
22). Unlike the clinical symptom measures used in the study, our measure of general adaptive functioning was not designed to support categorical diagnosis of any specific NDD and may therefore be more suitable for identifying neurobiological features that cut across different NDDs.
Adaptive functioning is most often measured in studies of ASD, wherein the level of adaptive impairment is not necessarily consistent with IQ or symptom severity among affected individuals (
38). Despite a prior call for imaging research in ASD that considers dimensional quantifiers, such as adaptive functioning, to better understand brain-behavior relationships in this disorder (
38), we are not aware of any imaging study that has examined the association of adaptive behavior with imaging measures in any NDD, nor of any studies that control for this variable. Recent work speaks to the importance of general adaptive functioning as a predictor of outcome in childhood NDDs (
39). Therefore, heterogeneous functioning in NDDs may serve as an important confounding factor to be considered in future studies. As found in a recent longitudinal study of outcome in ASD, improvement in adaptive functioning is not necessarily associated with improvement in clinical severity (
39). Novel interventions are needed to improve long-term outcome across NDDs. Our data suggest that intervention studies aimed at improving adaptive functioning may also investigate white matter fractional anisotropy as a mediating mechanism.
Although the present study is, to our knowledge, the largest pediatric imaging comparison of different neuropsychiatric disorders, our OCD and ADHD groups could still be considered moderately sized following rigorous quality control procedures, as necessary for pediatric neuroimaging studies (
40). Therefore, sample size and potential effects of unequal sample sizes may have limited the overall power of our study to detect other white matter alterations and brain-behavior relationships in NDDs. Furthermore, just under 30% of NDD participants in the current sample were medicated, and the pattern of medication use (with different neurochemical effects) varied in NDD groups. Although we controlled for medication status in the present study, analysis of larger samples enabling the examination of the specific effects of various medications and medication-by-diagnosis interaction effects on white matter are still needed. In addition, our results in NDDs may not generalize to age ranges outside of those that we studied. Future efforts to collect large longitudinal NDD data sets and larger numbers of female participants are needed to further resolve discrepant findings in the literature and extend understanding of developmental trajectories, the influence of IQ, and sex-specific findings in these disorders. It is noteworthy that in the present sample, multigroup comparison findings were maintained when a male-only analysis was conducted.