Research has shown that individuals diagnosed with schizophrenia experience cognitive decline from the premorbid to the postonset period. There is clear evidence for moderate cognitive deficits in children and adolescents who later develop schizophrenia, with meta-analyses showing an average premorbid deficit equal to 8 IQ points (SD=0.5) (
3,
4). Cognitive deficits in adults diagnosed with schizophrenia are more pronounced, with meta-analyses reporting a 14-point IQ deficit (SD=0.90) in first-episode schizophrenia patients (
5) and 15- to 21-point IQ deficits (SD=1.0–1.5) in chronic schizophrenia patients (
1,
6,
7). In line with cross-sectional evidence, longitudinal studies of cognitive change in schizophrenia from before to after illness onset have shown evidence for cognitive decline (
8). Three population-based studies have reported cognitive declines ranging from 6 to 12 IQ points (SD=0.4–0.8) between childhood and adulthood in individuals later diagnosed with schizophrenia (
8–
10).
Despite evidence for cognitive decline from before to after illness onset, the course of cognitive decline in schizophrenia remains unclear. While it is widely believed that cognitive impairments stabilize after illness onset (
11–
13), at least until older adult life (
12,
14), few longitudinal studies have examined cognitive change from illness onset through to a decade later (see Table S1 in the
online supplement), and findings across studies and cognitive domains are mixed. Studies have reported a stabilization of the cognitive deficits, cognitive decline, as well as amelioration of cognitive functioning (see Table S1; see also reference
15).
Previous studies have been unable to comprehensively chart the course of cognitive deficits, for several reasons. First, the majority of studies have used clinical samples, which may not be fully representative of the population of individuals with schizophrenia (
8). Second, most studies followed participants for only 1 to 3 years from illness onset (see Table S1). We previously reported a slow, gradual increase in premorbid cognitive deficits, with losses equal to between 0.5 and 1 IQ point per year (
16). Studies with short follow-ups, therefore, may be underpowered to capture decline. Third, few studies have included comparison groups, and therefore few have been able to consider the potential impact of normative age-associated changes in cognitive functioning, which is necessary to rigorously test for cognitive change. Since brain maturation continues into the third decade of life (
17), previous estimates of the magnitude of cognitive decline may be biased. Finally, few studies have examined the effect of medication on cognitive functioning, and yet recent findings suggest that antipsychotic medications may contribute to the severity of cognitive decline (
18).
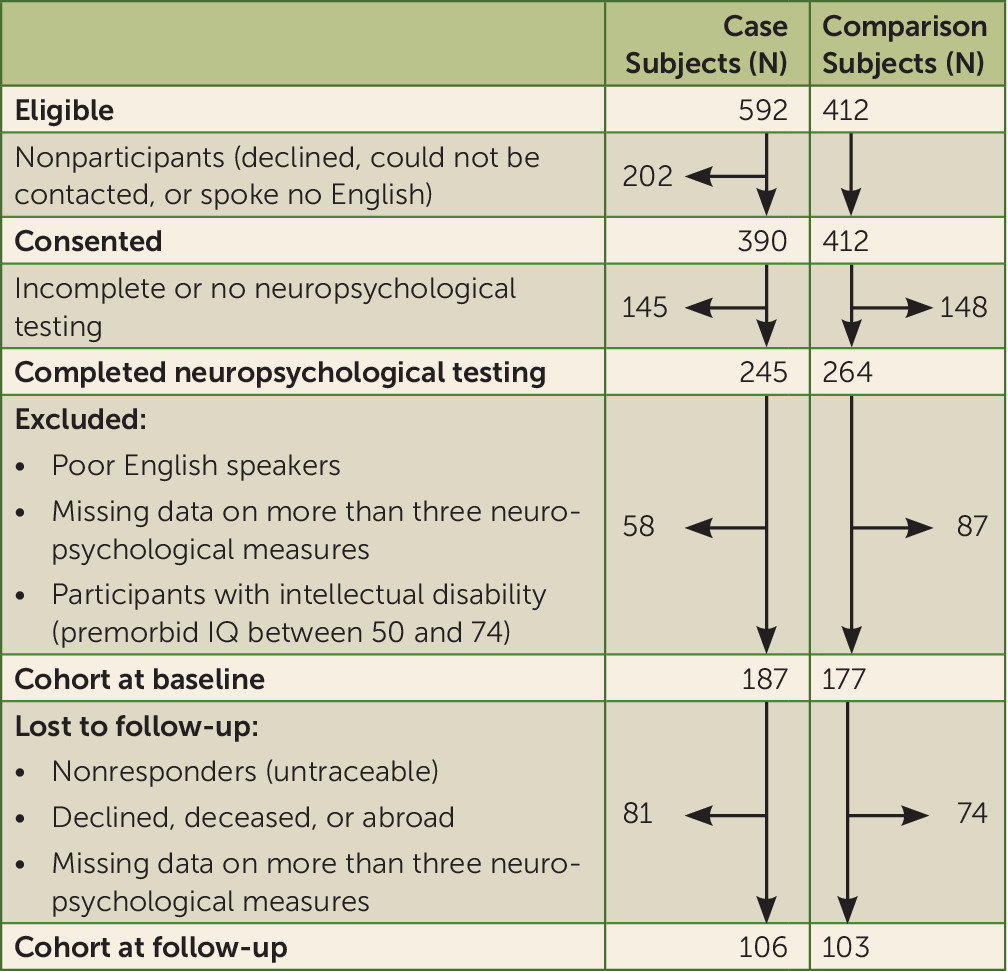
In a previous report on this population-based case-control study, we provided evidence for an IQ deficit as well as varying degrees of impairment across individual cognitive domains following the first psychiatric diagnosis of schizophrenia (
19). Study participants have since been followed up and have undergone neuropsychological testing a second time. Using identical neuropsychological measures at first assessment and follow-up, we were able to directly examine change in IQ and in individual cognitive functions after the first episode. To provide an accurate estimate of cognitive change over time, we compared patients to the healthy comparison subjects in the study who were followed during the same period. We tested three hypotheses. First, we examined the IQ decline hypothesis to establish whether schizophrenia patients exhibit a static IQ deficit or IQ decline. Second, we tested the generalized decline hypothesis to determine whether decline occurs across multiple cognitive domains, namely, verbal knowledge, memory, language, processing speed, executive function/working memory, and visuospatial ability. Finally, we tested the specificity hypothesis to establish whether any cognitive decline is specific to schizophrenia or common to other psychoses by examining cognitive change in individuals with psychotic disorders other than schizophrenia.
Discussion
Using a population-based case-control sample followed prospectively from the first psychotic episode, we provide evidence for cognitive decline after illness onset in patients with schizophrenia.
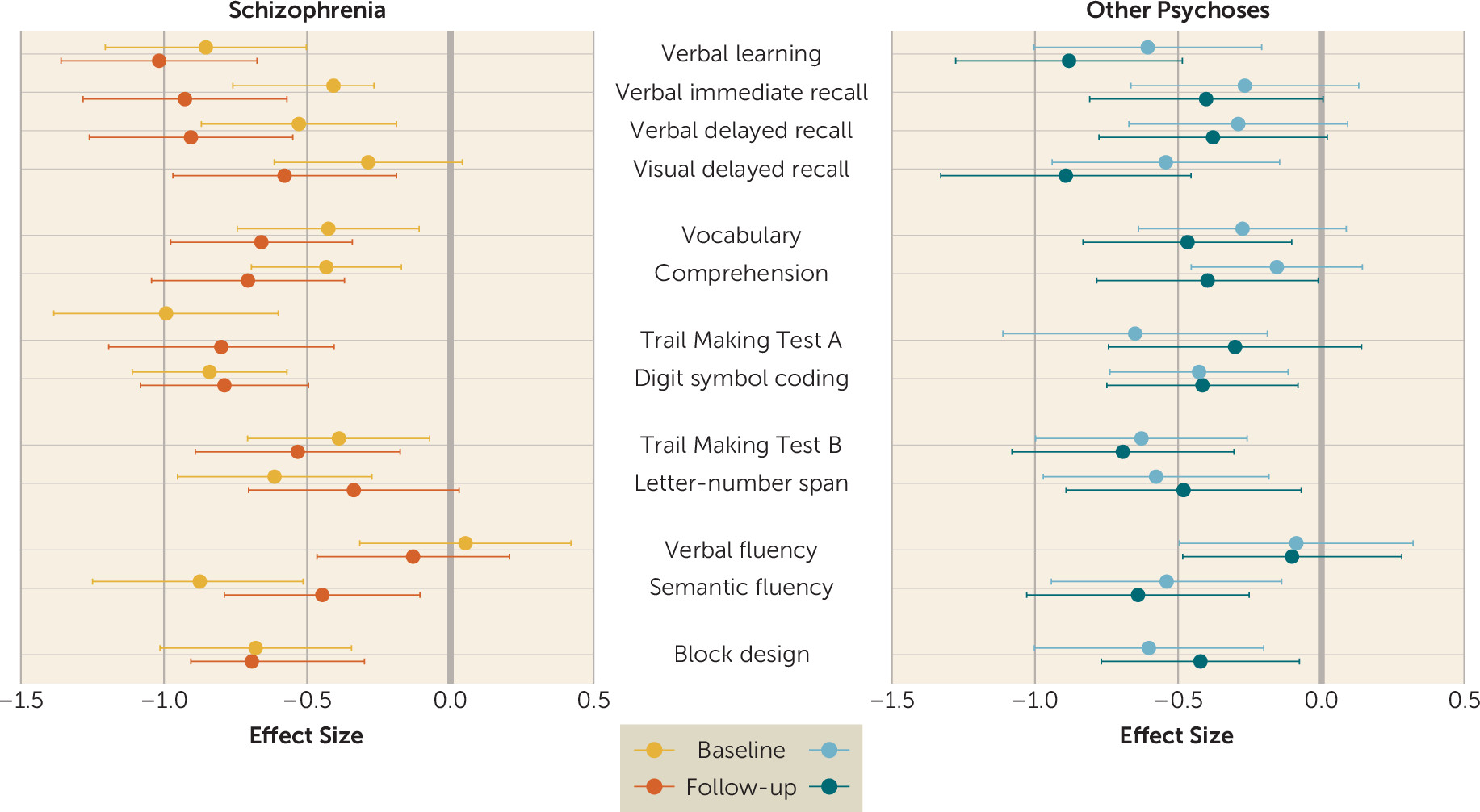
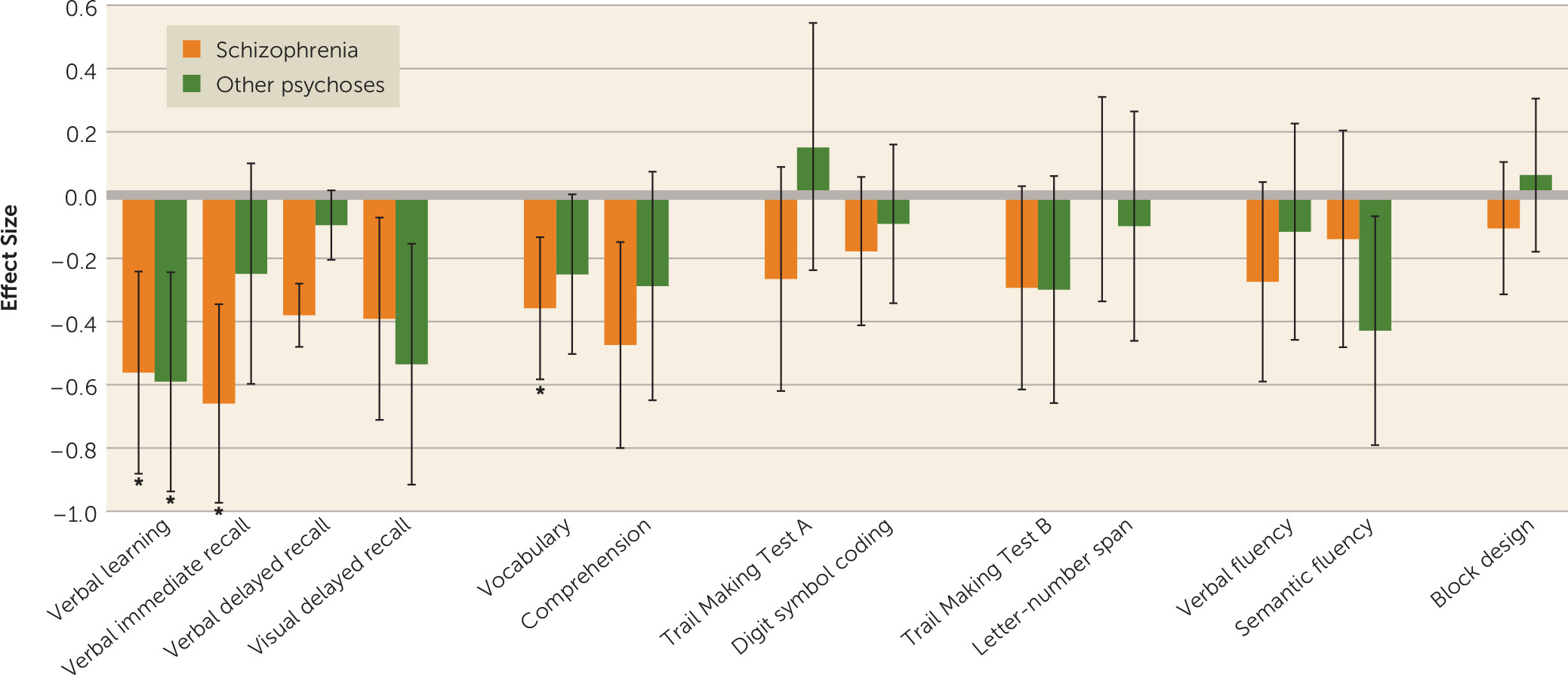
These findings advance knowledge in three important ways. First, the results lend support to the IQ decline hypothesis. As a group, schizophrenia patients showed IQ decline between baseline and follow-up assessments, with an effect size of small magnitude (0.28). This finding contrasts with earlier studies reporting stabilization of cognitive deficits after the onset of psychosis (
15). However, previous studies had important methodological limitations, including short follow-up periods and lack of a similarly followed-up comparison group. The finding of IQ decline is in line with findings from neuroimaging studies of greater age-associated brain volume loss (
34), as well as deviated gyrification trajectories in schizophrenia patients in adulthood (
35). Moreover, reduction in cortical volume has been associated with IQ decline in schizophrenia patients (
36).
Second, our findings here do not support the generalized decline hypothesis. Decline was not ubiquitous, and it varied across cognitive domains. The schizophrenia group exhibited declines in verbal knowledge and memory. In contrast, processing speed, executive functions, and visuospatial ability did not decline. These contrasts can be generally viewed as reflecting differences between the impact of the illness on crystallized (verbal knowledge) and fluid (processing speed, executive functions, visuospatial) abilities. Our findings of decreasing crystallized abilities and memory scores between baseline and follow-up are in line with previous evidence (
37) and suggest that increasing deficits in these domains may reflect actual loss of ability rather than abnormal cognitive development (i.e., “lag”) (
16). Alternatively, our findings may reflect difficulties with the maintenance and acquisition of new verbal knowledge as a result of substantial and increasing memory deficits. While most cognitive abilities in the general population start to show stabilization or even decline in early adulthood, crystallized abilities may peak much later (
38–
40). In our study, measures of fluid abilities already showed a large deficit at the first episode, which remained static thereafter. While previous longitudinal epidemiological studies have shown cognitive decline in schizophrenia from the premorbid period in childhood to the chronic stage in mid-adulthood (
8–
10), they were unable to determine when this decline occurred. Our findings suggest that most of the decline in fluid abilities occurs before the first episode, while crystallized abilities may continue to decline after onset. Importantly, the decline in IQ after onset is likely to be due to the decline seen in crystallized abilities.
Third, our findings do not support the specificity hypothesis, since patients with schizophrenia as well as those with other psychoses experienced cognitive decline. However, while patients with schizophrenia showed decline in IQ, memory, and verbal knowledge, patients with other psychoses showed decline only in certain memory functions. Moreover, in line with previous reports (
16,
41), the other psychoses group showed an overall impairment profile that was qualitatively similar yet quantitatively smaller than in the schizophrenia group. Thus, our findings suggest that cognitive decline is not specific to schizophrenia but is also evident in other psychoses. However, large, widespread cognitive decline may still be specific to schizophrenia, since the other psychoses group showed a smaller and less generalized cognitive decline. Interestingly, there was no evidence of decline in a key comparison group, namely, individuals with lower IQ who did not develop psychosis. This group may in fact experience a different process of regression to the mean.
Our findings in this study should be viewed in the context of certain limitations. First, although we found evidence for cognitive decline after illness onset, we could not fully map the course of deficits, and cognitive functions may vary in the timing of decline after the first episode. Second, group sizes did not allow for an analysis of the heterogeneity of cognitive course and also limited our ability to investigate more specific diagnostic subgroups, such as bipolar disorder and mania. Third, we ruled out two explanations for the observed cognitive decline, namely, type and duration of antipsychotic treatment. Unfortunately, we did not have information to examine other potential moderators of cognitive decline, such as social isolation, smoking, illicit drug abuse, victimization, or physical health problems such as obesity, diabetes, and hypertension. Moreover, despite the fact that we adjusted for education in all our analyses, poor education in the schizophrenia group after the first psychotic episode could still partly explain some of the group differences.
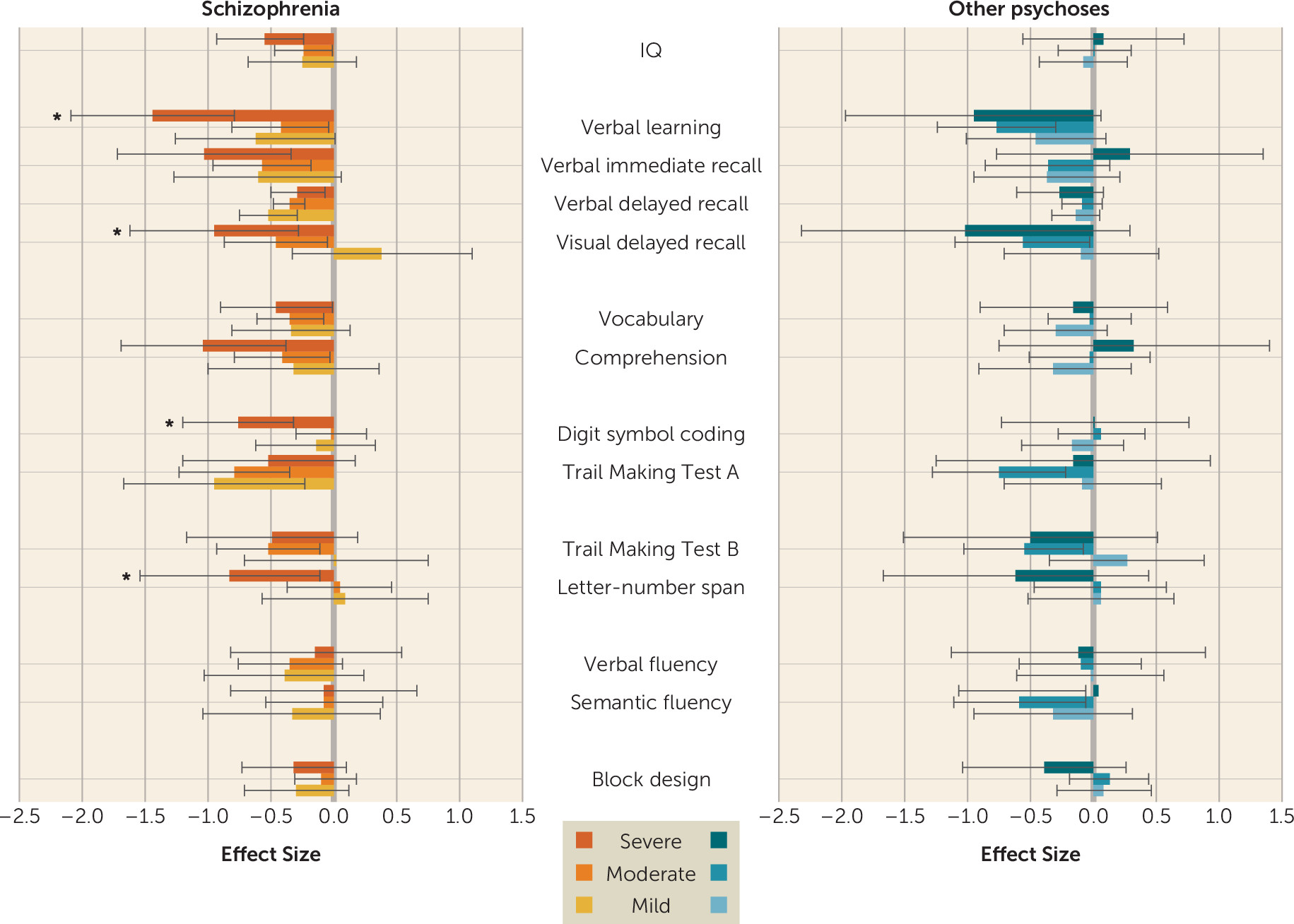
There is conflicting evidence regarding the relationship between change in symptoms and cognitive functioning (
42,
43). In our study, change in severity of psychosis was only minimally associated with cognitive change. These results are consistent with cross-sectional findings of only a weak association between positive symptoms and cognitive impairment (
44). Longitudinal evidence also suggests a minimal association between change in positive as well as negative symptoms and change in cognition (
42–
45). Interestingly, in our study, schizophrenia patients with severe symptoms at baseline showed greater cognitive decline than patients with mild or moderate symptoms. While this subgroup was small (21% of the overall group), the magnitude of decline in the memory domain was large. Thus, this finding points to a potential subgroup of schizophrenia patients who may greatly benefit from being specifically targeted for cognitive remediation.
Our findings have important implications for understanding the nature and course of cognitive impairment in schizophrenia, as well as in other psychoses. Integrating the present findings with those of previous studies (
16) suggests that cognitive dysfunction in schizophrenia may result from a complex interplay between an early, static neuropathology (
46,
47) and dynamic age-related processes (
48,
49). Hence, cognitive functions that develop and peak relatively early in life, such as processing speed and visuospatial abilities (
39), may show aberrant development, resulting in slowed growth before the onset of schizophrenia (
16), but relative stabilization throughout the illness course. On the other hand, cognitive functions that continue to evolve through adult life, such as language (
39), may show further deterioration throughout the course of schizophrenia. Finally, functions sensitive to age-related cognitive decline, such as memory, may begin to decline in middle adulthood, before normative aging becomes apparent (
40).
In conclusion, this study demonstrates that while a substantial proportion of the cognitive impairment seen in adult patients with schizophrenia, as well as in other psychoses, is already present at the first episode, these patients continue to experience cognitive decline after illness onset. However, the nature of this decline varies across neuropsychological functions. While large deficits in processing speed are already apparent at the first episode, deficits in verbal knowledge and memory continue to increase. These findings suggest that different pathophysiological mechanisms may underlie individual neuropsychological deficits seen in adult psychosis patients. Future research should determine which of these are consequent to the illness itself, and which to the psychosocial factors patients experience. Finally, these findings highlight the importance of targeting early developmental stages in future studies of the causes of cognitive deficits associated with psychosis, as well as in cognitive remediation efforts.