Schizophrenia is a severe psychiatric disorder that emerges most commonly in late adolescence and early adulthood, affecting approximately 1% of the population (
1). Recent studies have focused on detection and treatment intervention in the early stages of psychotic illness (
2), especially during the clinical high-risk phase, before a full-blown disorder is present (
3,
4). Because psychotic symptoms, cognitive dysfunctions, and disturbances in social functions may all evolve in the clinical high-risk phase (
5–
8), investigating brain alterations in clinical high-risk individuals could potentially shed light on their underlying mechanisms.
Studies in the past two decades, and especially those applying diffusion MRI (dMRI) techniques, strongly suggest white matter abnormalities in schizophrenia, where disrupted cortico-cortical connections may play a key role in both clinical symptoms and cognitive dysfunctions (
9–
13). Most studies apply diffusion tensor imaging (DTI) (
14,
15) to estimate fractional anisotropy (FA), a measure sensitive to the arrangement and microstructure of white matter, often used to assess white matter integrity (
9,
12,
13,
16). Lower FA, however, may be caused by different factors, including axon diameter alterations, alterations in myelin sheath, increased extracellular volume fraction, and alterations in the organization of axonal fibers (
17–
23). Consequently, FA provides a nonspecific and less than optimal estimate of white matter integrity (
24), making it hard to relate FA changes to a specific underlying pathology. In addition, meta-analysis has shown that FA alterations may be affected by illness stage and by exposure to antipsychotic or antidepressant medications (
25). Thus, additional methodological advances and a careful selection of study populations are required to determine the source(s) of white matter alterations and their association with illness progression.
One way to increase FA specificity is to correct for partial volume effects with extracellular water. For example, free-water imaging analysis (
19,
23,
26) uses two compartments to separately model diffusion properties of brain tissue and those of surrounding free water, which can be found only in the extracellular space (
19). Fractional anisotropy of cellular tissue (FA
T), which is more specific to white matter cellular alterations than conventional FA measures, is derived from the tissue compartment after the elimination of the free-water contribution. In addition, this model estimates the fractional volume of the free-water compartment (FW), which is affected by processes that change the extracellular space, such as atrophy and neuroinflammation (
18,
27). FW maps can also be estimated using other dMRI techniques (e.g.,
17,
28,
29), and across methods, they show high signal in CSF-filled cavities and lower, albeit nonzero, signal throughout the white matter.
Previous free-water imaging studies comparing patients after the first psychotic episode with healthy control subjects have shown widespread higher FW with limited focal lower FA
T in frontal lobe regions of patients (
18,
30). In chronic patients, the reverse has been noted—that is, a greater extent of significantly lower FA
T with less extensive significantly higher FW (
27,
30). These results suggest that white matter abnormalities may be explained by co-occurring alterations: extracellular free-water alterations, measured by FW, and microstructural cellular alterations, measured by FA
T. The higher FW may thus reflect an acute brain response to psychosis, which is less pronounced in chronic stages, while lower FA
T may reflect preexisting neurodevelopmental abnormalities and/or a continuous process of accumulating cellular damage.
An important step toward understanding the source of these different white matter alterations is to study clinical high-risk individuals to determine whether or not extracellular and cellular alterations are present before the onset of psychosis, and if so, to what extent. There have been only a limited number of dMRI studies in clinical high-risk samples, most of which were based on DTI, and most of which had small sample sizes. Two separate white matter fiber tracking studies (
31,
32) reported no significant differences between clinical high-risk individuals and healthy control subjects. In contrast, three other studies showed limited frontal regions with lower FA (
33–
35). In two additional tract-based spatial statistics (TBSS) studies (
36,
37), extensive regions with lower FA were observed in the corpus callosum, the superior longitudinal fasciculus, the internal capsule, and the corona radiata of clinical high-risk individuals. In one study that applied free-water imaging in a clinical high-risk cohort, group differences were found in FA
T in fibers connecting the salience network (
38), but FW has not been tested. These studies, taken together, suggest that although white matter alterations are likely present in clinical high-risk individuals, they are inconsistent, may not have a distinct location, and are more subtle than those observed in schizophrenia.
In this study, we went beyond previous studies by performing a dMRI study on a large clinical high-risk sample that was recruited in China, where most individuals were naive to psychotropic medications. Furthermore, we used free-water analysis to separately evaluate the extent and putative roles of extracellular and tissue-related microstructural white matter. Based on our first-episode study, we predicted that both types of alterations (higher FW and lower FAT) would be present in the clinical high-risk stage. In addition, based on the extent of the alterations, we sought to identify which of these processes, extracellular or cellular changes, are more related to attenuated symptoms prior to onset of psychosis.
Results
Demographic and Clinical Characteristics
There were no significant differences between the clinical high-risk and healthy control groups in age, gender, education, motion, or handedness (see
Table 2). Clinical high-risk individuals experienced attenuated positive symptoms (see
Table 1), manifested primarily as elevated symptoms on P1 (unusual thought content/delusional ideas) and P2 (suspiciousness/persecutory ideas) of the SOPS. The mean duration of prodromal symptoms at assessment was 6.5 months (SD=6.2). Forty of the 50 clinical high-risk individuals had never been treated with antipsychotics or antidepressants. Ten clinical high-risk individuals had been treated with antipsychotic or antidepressant medications for less than 3 weeks (aripiprazole, N=3; risperidone, N=2; quetiapine, N=1; sulpiride, N=1; citalopram, N=1; and olanzapine, paroxetine, and lamotrigine, N=1), and one had been treated with an antipsychotic for 3 months (risperidone). The 10 medicated participants (eight of them male; mean age, 19.3 years [SD=3.7]) had significantly more functional decline than the nonmedicated participants (t=2.12, df=48, p=0.04); they also had more negative symptoms, although the difference fell short of significance (t=1.78, df=48, p=0.08). There were no other significant differences in demographic or clinical characteristics between the medicated and nonmedicated clinical high-risk individuals (see Table S1 in the
online supplement).
Within the 1-year follow-up evaluation, 11 clinical high-risk individuals (seven of them male; mean age, 18.4 years [SD=2.2]) converted to psychosis and were diagnosed with schizophrenia. The average time between initial assessment and conversion was 6.27 months (SD=5.96, range=1–17). Nine of those who converted to psychosis had never been medicated at baseline. There were no significant differences in age, gender, or clinical scores between clinical high-risk individuals who converted to psychosis and those who did not.
Whole Brain Average Diffusivity Comparison of Clinical High-Risk and Healthy Control Groups
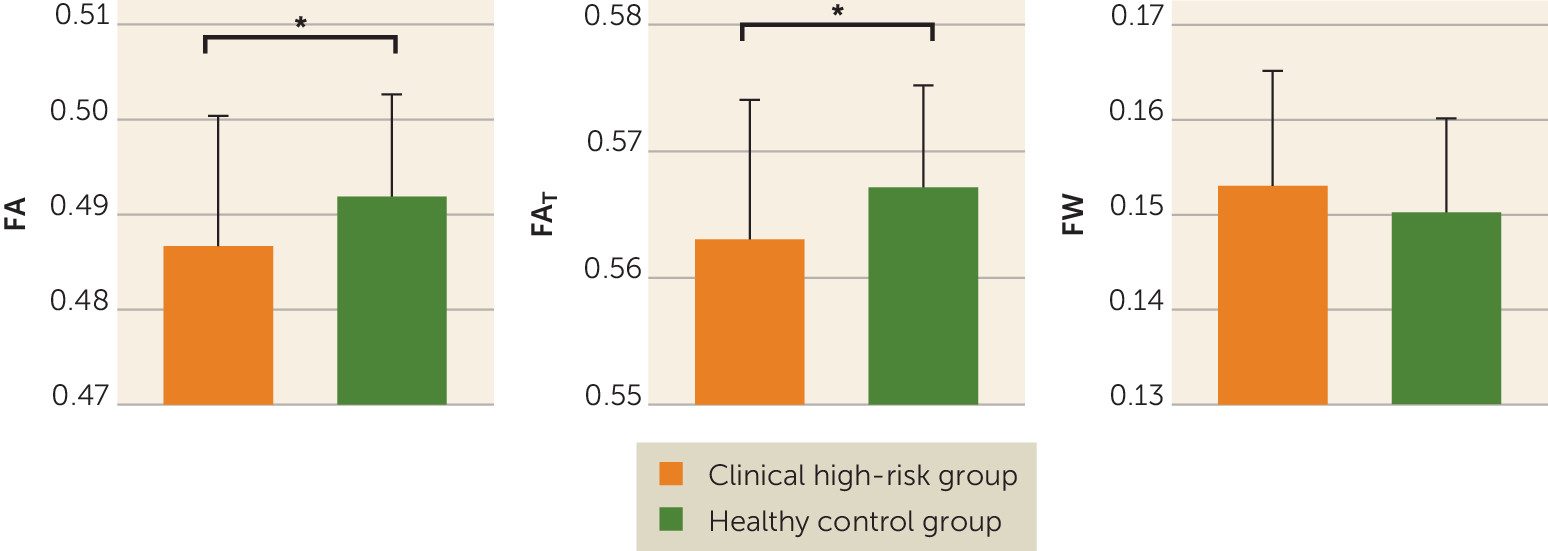
In comparing average diffusivities across the entire white matter skeleton, we found that the clinical high-risk group had significantly lower FA compared with the healthy control group (0.487 [SE=0.002] and 0.492 [SE=0.002], respectively; F=5.672, df=1, 95, p=0.019; Cohen’s d=−0.422) (
Figure 1). When applying the free-water model, we found that the clinical high-risk group had significantly lower FA
T than the healthy control group (0.563 [SE=0.002] and 0.567 [SE=0.001], respectively; F=5.556, df=1, 95, p=0.020; Cohen’s d=−0.424) but not significantly higher FW (0.153 [SE=0.002] and 0.150 [SE=0.001], respectively; F=2.243, df=1, 95, p=0.137; Cohen’s d=0.034) (
Figure 1). The lower FA
T coincided with lower AD
T (F=6.348, df=1, 95, p=0.013; Cohen’s d=−0.499) but not with higher RD
T (F=1.295, df=1, 95, p=0.258; Cohen’s d=0.164). Additionally, FA
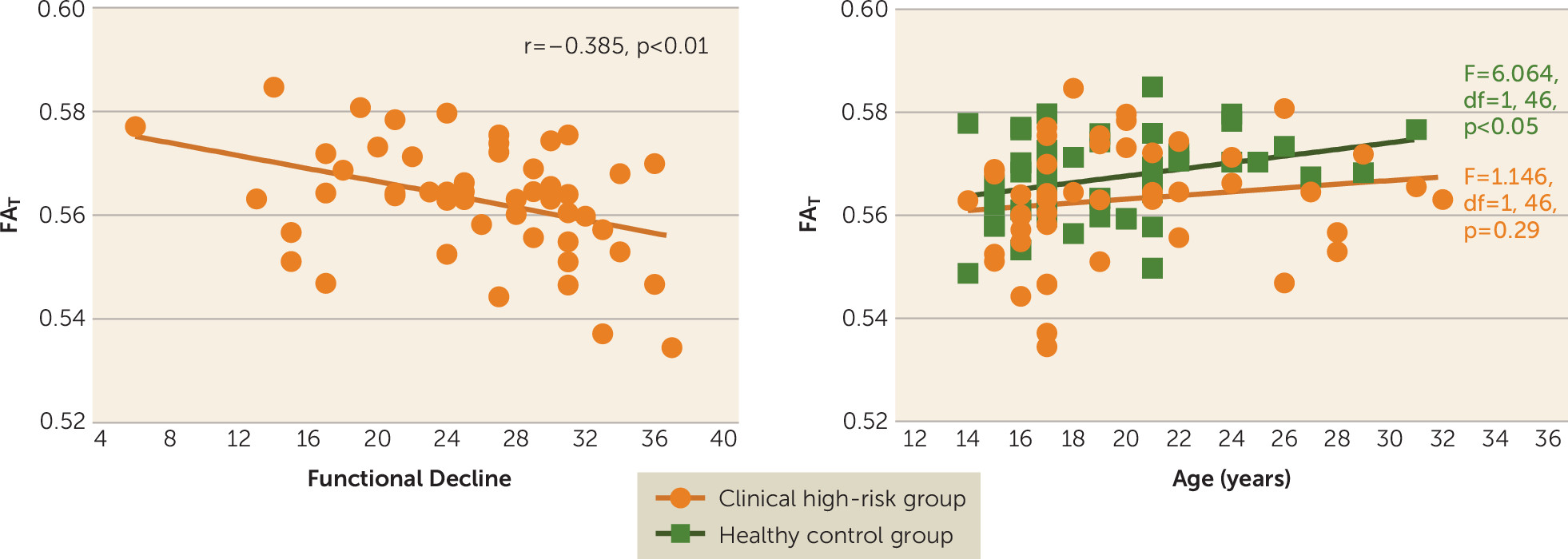
T was negatively correlated with functional decline in the clinical high-risk group (r=−0.385, df=48, p=0.006) (
Figure 2); that is, individuals with lower FA
T tended to have more functional decline. Significant correlations with functional decline were also found for the AD
T measure (r=−0.299, df=48, p=0.035) and the RD
T measure (r=0.300, df=48, p=0.034). The uncorrected FA measure was also correlated with functional decline, although to a lesser degree than FA
T (r=−0.357, df=48, p=0.011). There was no significant correlation between FW and functional decline (r=0.161, df=48, p=0.265). There were also no significant correlations between the diffusion measures (FA, FA
T, AD
T, RD
T, and FW) and any other clinical score.
Gender (F=4.337, df=1, 95, p=0.040) and age (F=5.951, df=1, 95, p=0.017) were significant predictors of FAT across the entire sample. There was no significant interaction of group and gender (F=1.706, df=2, 97, p=0.187). However, for FAT there was a significant interaction of group and age (F=4.789, df=2, 97, p=0.010), suggesting age-related differences between groups. Follow-up linear models within the healthy control group showed that FAT was positively correlated with age (F=6.064, df=1, 46, p=0.018). However, in the clinical high-risk group, FAT was not correlated with age (F=1.146, df=1, 46, p=0.290).
There were no significant differences between nonmedicated clinical high-risk individuals and those who received antipsychotics or antidepressants on either of the free-water measures (for FAT, F=2.003, df=1, 45, p=0.164; Cohen’s d=0.425; and for FW, F=0.051, df=1, 45, p=0.822; Cohen’s d=0.018). For the nonmedicated clinical high-risk group, correlation with functional decline remained significant (r=−0.397, df=38, p=0.011), although the decrease in FAT compared with the healthy control group fell short of significance (F=2.931, df=1, 85, p=0.091; Cohen’s d=−0.278). There were no significant group differences in FAT (F=2.078, df=1, 45, p=0.156; Cohen’s d=0.423) or FW (F=0.006, df=1, 45, p=0.939; Cohen’s d=0.128) between clinical high-risk individuals who converted to psychosis and those who did not.
Voxel-Wise Analyses
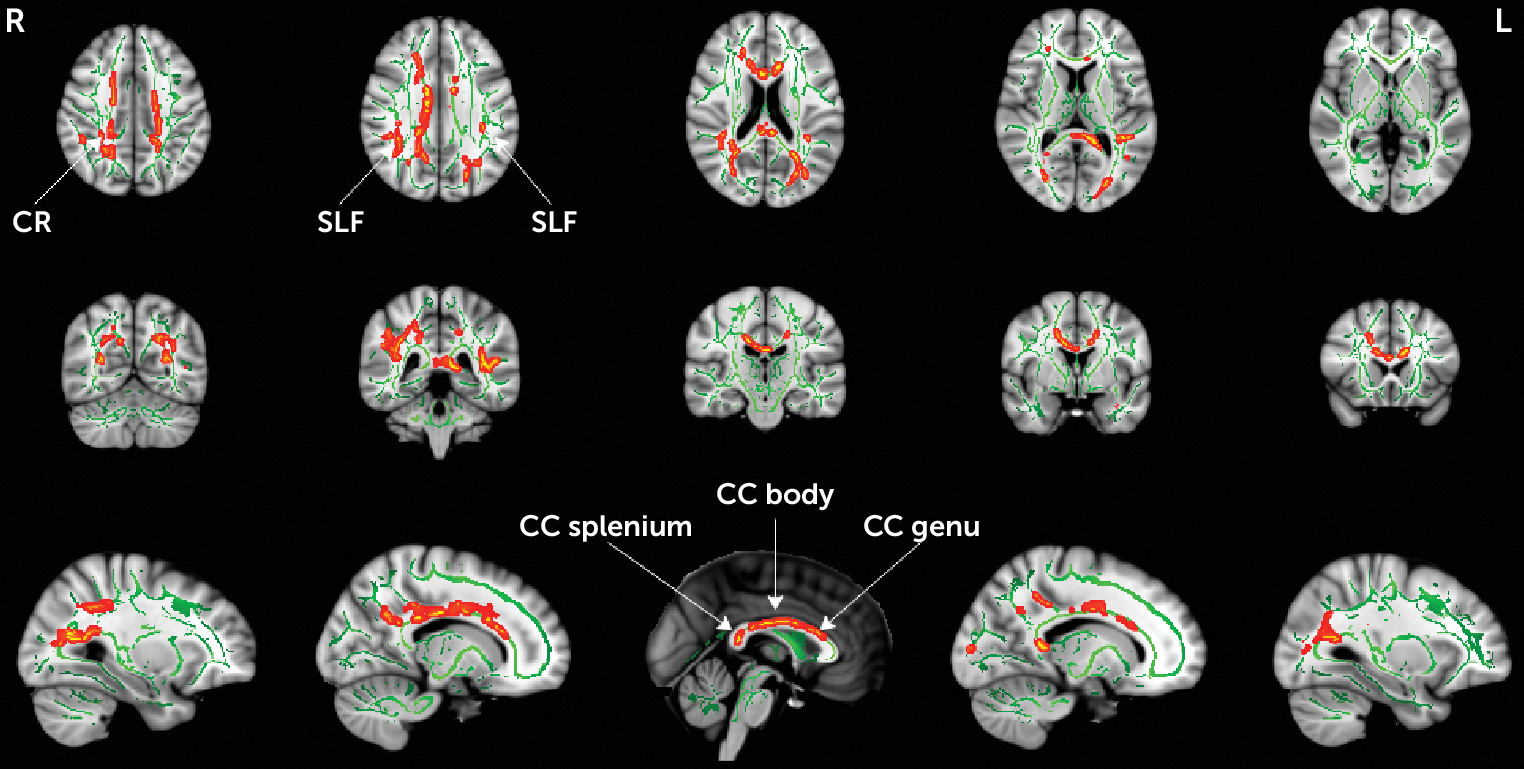
To better understand the extent of microstructural alterations, FA
T and FW were compared between the clinical high-risk and healthy control groups using a voxel-wise TBSS analysis. Similar to the whole brain analysis above, there were no group differences in FW. The statistical analysis identified lower FA
T in the clinical high-risk group in 4% of the skeleton (5,363 voxels), and in the following locations: the genu, body, and splenium of the corpus callosum; the right anterior, superior, and posterior corona radiata; and the left and right superior longitudinal fasciculus (
Figure 3). These locations largely overlapped with the group differences in FA (see Figure S1 in the
online supplement), which covered 10.6% of the skeleton (14,267 voxels). There were no voxel-wise correlations between FA
T and clinical parameters. However, there was a significant correlation (r=−0.405, df=48, p=0.004) between functional decline and FA
T averaged across all voxels that showed significant FA
T group differences. There were no significant voxel-wise group differences in AD
T or RD
T. Also, we did not identify any significant group differences between clinical high-risk individuals who converted to psychosis and those who did not.
Discussion
We report differences in the white matter of a large and predominantly medication-naive cohort of clinical high-risk individuals compared with healthy control subjects. We demonstrated that the abnormalities observed in the clinical high-risk group originate from cellular (FA
T) rather than from extracellular differences (FW) in the brain. This is in contrast to previous findings in first-psychotic-episode patients who presented with a large extent of extracellular differences in addition to cellular differences (
18,
30). We further note that the cellular differences are manifested as an abnormal age-related effect and are associated with functional decline. We note, however, that our analyses assumed a linear relationship of the diffusion parameters with age and were based on cross-sectional data. More accurate longitudinal models that are based on larger samples are needed to better characterize the relationship with age, as well as group differences in age-related effects. Our findings thus suggest that early cellular alterations appear prior to psychosis onset in clinical high-risk individuals experiencing attenuated psychotic symptoms.
The finding of lower FA
T is in agreement with previous studies that have observed lower FA and FA
T in clinical high-risk individuals (
36–
38). FA
T is meant to improve the specificity of the measurement to processes that occur within or around neuronal tissue. FA
T is expected to show less difference between groups than FA when much of the group differences originates from extracellular processes (e.g.,
18,
30,
51). On the other hand, in some instances, extracellular processes may dominate the signal but may not show differences between the groups, in which case FA
T would show more findings than FA (e.g.,
52). Finally, if there is not a large contribution to the signal from extracellular processes, FA
T is expected to be similar to FA (
19). Our study goes further, therefore, in that it helps establish that microstructural abnormalities, which are already apparent in the clinical high-risk phase, likely originate from the tissue itself and not from extracellular free water. Of further note, in this study, the exact underlying pathology related to cellular alterations is difficult to determine. Nevertheless, the cellular alterations were correlated with more functional decline over time, rather than with current symptom levels, which may suggest an ongoing deterioration. This correlation remained significant after medicated clinical high-risk individuals were excluded, which provides reassurance that this is not a medication effect. In addition, the positive correlation between FA
T and age in the healthy control group, which was not observed in the clinical high-risk group, suggests an abnormal age-related effect in the clinical high-risk group.
Within the age range of our sample, many of the white matter bundles are still at a stage of development before reaching a peak in anisotropy (
53). The lack of a positive correlation between FA
T and age in the clinical high-risk group may therefore suggest that white matter fiber development in the clinical high-risk individuals was prematurely disrupted, which possibly points to a neurodevelopmental anomaly. Also supporting this hypothesis is that FA
T changes were associated with AD
T changes, which are thought to be more related to axonal organization, and not with RD
T changes, which are thought to be more related to neurodegeneration or demyelination (
54). Taken together, the FA
T group differences raise the possibility that initial preexisting cellular anomalies confer a predisposition for the development of symptoms and that any subsequent cellular deterioration determines the severity of the functional decline experienced by the clinical high-risk individual. This possibility could be tested by obtaining longitudinal imaging in clinical high-risk individuals prior to onset of psychosis.
The location of FA
T group differences included the genu, body, and splenium of the corpus callosum; the right anterior, superior, and posterior corona radiata; and the left and right superior longitudinal fasciculus. These differences found in a Chinese sample are in line with previous whole-brain studies of clinical high-risk cohorts (
33,
34,
36,
37). In particular, white matter abnormalities within the superior longitudinal fasciculus, which connects the fronto-parieto-temporal regions, are the most frequently reported abnormalities in clinical high-risk individuals (
33,
34,
36). Interestingly, we report more locations with FA
T group differences than in our previous studies of first-episode patients with psychosis (
18,
30). Since it is not likely that cellular changes diminish with the progression of the disorder, we believe that several differences in the design of the present study likely led to higher sensitivity for identifying FA
T group differences. These design differences include a larger sample size, which increases the study’s power to identify group differences, and an improved multishell acquisition method, which provides better signal-to-noise ratio and a more robust model fit. The higher sensitivity of the multishell sequence can also be appreciated by noting the higher spatial extent of FA
T group differences in this study compared with a previous single-shell study with a similar population (
38).
Of particular note, based on our previous studies of patients after their first psychotic episode (
18,
30), we predicted that FW would be higher in the clinical high-risk group. However, we did not find extracellular FW group differences in the present clinical high-risk sample. Since this study is better powered and uses a more sophisticated diffusion MRI sequence than previous studies, the lack of FW findings suggests that FW changes are either absent or much more subtle than those observed in first-episode patients. Thus, this study suggests that substantial FW alterations may appear closer to psychosis onset, as opposed to the present clinical high-risk sample, in which psychosis was not present. The absence of higher FW in clinical high-risk individuals substantiates a hypothesis that an increase in FW likely represents an acute response of the brain to psychosis and that the increase reaches a peak after the first psychotic episode and then declines in the chronic phases (
27). Such a hypothesis could be tested in future studies, especially by following clinical high-risk individuals longitudinally, where an increase in FW is predicted in those individuals who convert to psychosis compared with those who do not. Relating increased FW with acute response to psychosis may lead to treatment approaches that may prevent such an increase at the clinical high-risk stage (e.g., anti-inflammatory treatments), which may also prevent the outbreak of psychosis.
Limitations of this study include the cross-sectional nature of the analysis and the fact that the sample was not large enough for us to identify differences between participants who developed psychosis and those who did not. Clinical high risk is an early, potentially recoverable stage of psychotic disorder (
55), and thus it could be argued that the clinical high-risk state may not be representative of psychosis. However, all clinical high-risk individuals in our sample experienced attenuated psychotic symptoms, and it may be that conversion to psychosis is determined by the interaction of neuropathology with external factors and life events, quality of the support system, environmental factors, treatment strategies and response to treatment, or supervening pathophysiological processes, such as neuroinflammation, that add to an already existing biological vulnerability (
56). Future assessments in a large, longitudinal sample of clinical high-risk individuals who converted to psychosis will be important in validating our findings in this study. While our cohort in this study was largely drug naive, our results suggest that group differences may be attributed in part to medication status or may be a proxy of symptom severity. Studies with a sufficient number of medicated and nonmedicated clinical high-risk individuals are needed to establish whether or not medication has an effect on the diffusion MRI measures. We note that while this cohort was recruited in China, we used the same procedures and protocols as in our other studies with cohorts in the United States. Therefore, we do not expect technical differences to affect our results, although genetic differences between Chinese and Western cohorts may contribute to some of the differences between this and previous studies. The specific multishell sequence that we used here was designed to support the model-based quantitative analysis in addition to tractography studies. Free water is visible in the low b-value range, and here we added two lower shells. In low b-values, the signal profile is isotropic and therefore requires fewer gradient orientations, although the acquisition could be further optimized in future work. We note that the high b-value shell was not used in this study because it is not appropriate to include in a Gaussian diffusion model such as the free-water imaging. The information in the high b-value shell awaits further work with non-Gaussian models or tractography studies.
In conclusion, our data indicate that microstructural white matter alterations are present in clinical high-risk individuals who are experiencing attenuated psychotic symptoms, prior to the onset of psychosis, and that these alterations are associated with functional decline, as well as possibly reflecting a predisposition to develop attenuated psychotic symptoms. In contrast, higher extracellular FW was not observed in this clinical high-risk sample, suggesting that previously reported extracellular processes, such as neuroinflammation, may play a more prominent role closer to or at onset of psychosis. As we follow this sample longitudinally, we will be able to provide important new information bearing on the relationship between white matter changes and the emergence of psychosis.