Modern antipsychotic medications fail to resolve clinical symptoms in approximately a third of people with schizophrenia, a condition known as treatment resistance (
1). For several decades, this clinical challenge has motivated attempts to develop more effective next-generation antipsychotics (
2–
6). However, research has been hindered by insufficient understanding of the neurobiological mechanisms of and lack of valid brain biomarkers for treatment resistance. Current antipsychotic drugs share their neurotransmitter targets in dopaminergic systems. Their ineffectiveness in patients with treatment-resistant symptoms suggests that other mechanisms and neurotransmitter systems may be involved, although the evidence is still preliminary (
7–
10). Some structural neuroimaging studies suggest that treatment resistance is associated with reduced gray matter and cortical thickness (
7,
11,
12). However, a meta-analysis found no replicated neuroimaging findings when comparing patients with treatment-resistant schizophrenia with patients whose clinical symptoms were treatment responsive (
13).
Patients with treatment-resistant schizophrenia share two consistent features: earlier age at illness onset and more severe cognitive deficits (
14–
17); this suggests both neurodevelopmental and cognitive contributions to treatment resistance. Therefore, brain measures that track with neurodevelopmen and cognitive dysfunctions in schizophrenia may help identify biomarkers of treatment resistance. White matter shows promise as a research focus in schizophrenia, and the largest meta-analytic study of white matter deficits in schizophrenia to date, conducted by the Enhancing Neuro Imaging Genetics Through Meta-Analysis (ENIGMA) consortium, determined a region-specific pattern of white matter deficits (
18). The pattern of regional differences found in the ENIGMA study confirmed findings from previous diffusion tensor imaging (DTI) studies that demonstrated significant deficits in the frontal associative white matter regions. This included the anterior corona radiata and the genu and body of the corpus callosum (
19–
23). The pattern indicated in the ENIGMA study was also in line with histological findings of reduced glial cell density and myelination in the frontal lobe in patients with schizophrenia (
24–
26). This regional white matter deficit pattern as identified in the ENIGMA study contributes to core cognitive dysfunctions, especially in processing speed deficits in schizophrenia (
27–
30). Interestingly, among all of the cognitive abnormalities associated with treatment resistance, the largest deficit is observed in processing speed (
16). Both white matter and processing speed development follow an inverse-U neurodevelopmental trajectory, and the peak of myelination for associative white matter overlaps with the peak of processing speed abilities (
31,
32). The origin of the white matter deficit pattern in schizophrenia is not fully understood, but the disorder-related alterations in development that prevents normal development of late-myelinating areas likely prevents the establishment of the normal pattern of interneuronal communication (
33). This may lead to the observed contrast in regional deficits between late- and early-myelinating white matter regions in schizophrenia, as observed in the ENIGMA results and in other studies (
18,
34,
35). Therefore, we hypothesized that the regional white matter deficit pattern may represent a neurobiological mechanism that leads to treatment resistance in schizophrenia. To test this hypothesis, we assessed whether white matter regional deficits index treatment resistance by comparing patients with treatment-resistant schizophrenia with patients whose symptoms were treatment responsive. In parallel, we compared regional deficits in patients who underwent imaging within 2 weeks of the initiation of antipsychotic treatment with healthy control subjects. We used this comparison to control for potential chronic antipsychotic medication effects on white matter deficits and to determine whether the identified white matter deficits were also associated with schizophrenia, independent of chronic disease and treatment courses. Evidence supporting this hypothesis would implicate white matter deficits as a contributing factor of treatment resistance.
Discussion
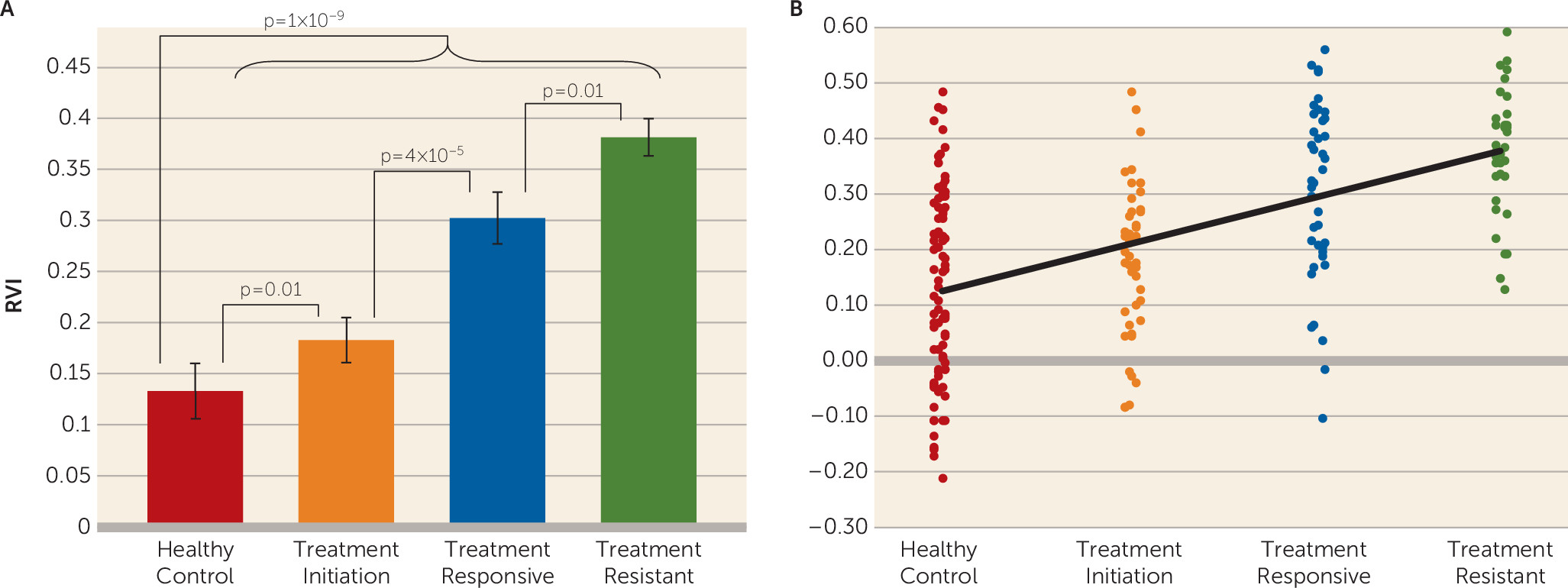
We found that the white matter regional deficit pattern in schizophrenia was significantly associated with treatment resistance. The white matter RVI significantly differentiated patients with treatment-resistant schizophrenia from patients with treatment-responsive schizophrenia, even with matched treatment duration and in the absence of global FA differences. The RVI measure further significantly differentiated patients at treatment initiation from healthy control subjects. These results suggest that the extent of regional white matter vulnerability, as defined by the RVI, can be observed in schizophrenia at initial diagnosis and treatment and may mark the liability for treatment resistance to the currently available antipsychotic medications. Follow-up longitudinal studies will be required to test whether higher RVI at the onset would track with the development of treatment resistance.
Treatment resistance has been linked to cognitive deficits, especially in processing speed (
15,
16) and negative symptoms (
14), although the underlying mechanisms remain unknown (
28–
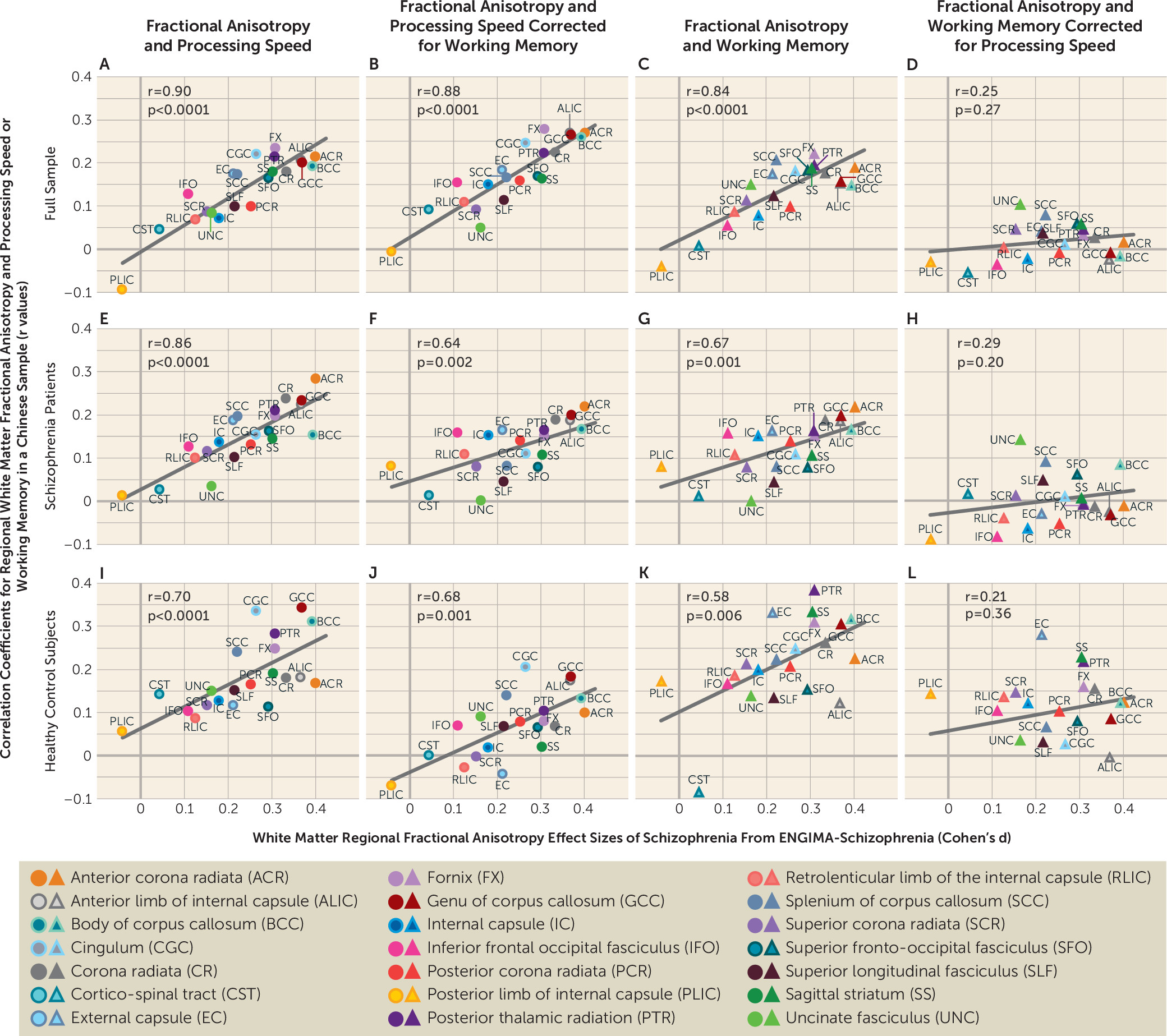
30). Data from this study suggest that white matter may represent a shared underlying neurobiology, because the regional vulnerability pattern appears to be associated with treatment resistance and with more severe negative symptoms. We also replicated findings from a previous study in which the schizophrenia pattern in the ENIGMA meta-analysis predicted an association between FA and processing speed and working memory (
27) (
Figure 3; see also Figure S1 in the
online supplement). That study employed only two cognitive tasks, which was considered a limitation (
27). In the present study, we used the MCCB to replicate these findings, and the similar pattern across two diverse (U.S. and Chinese) samples further validates white matter regional vulnerability in indexing core cognitive deficits in schizophrenia.
A white matter association with treatment resistance was initially suggested by white matter volume reduction findings (
43), but opposite results have also been reported (
11). Several DTI studies further suggested a white matter effect in patients with treatment-resistant schizophrenia (
44,
45). However, without a direct comparison using sufficient sample sizes with age-, sex-, and treatment duration-matched patients who are treatment responsive, it is difficult to interpret whether these previous findings were related to schizophrenia in general or were specific to treatment-resistant schizophrenia. We showed that multiple white matter regions had robust and significant reductions in FA in schizophrenia patients compared with healthy control subjects (see Table S1 in the
online supplement), which is consistent with many previous studies (
19–
23,
27,
46,
47). No individual white matter region could consistently differentiate patients with treatment-resistant schizophrenia from patients with treatment-responsive schizophrenia after correction for multiple comparisons. Why the RVI but not the individual regional measures captured treatment resistance (by comparison with treatment response) is not immediately clear. RVI is a readily obtainable index that correlates normalized regional FA of each person to the schizophrenia effect sizes in the ENIGMA study and is assumed to reflect the contrast between the high vulnerability to schizophrenia of late-developing associative white matter regions and the lower vulnerability of early-developing regions (
34,
48,
49). We speculate that by taking into account white matter across the whole brain, the RVI may have reduced nonspecific effects that affect all white matter regions and further accentuated the regional effects specific to schizophrenia (
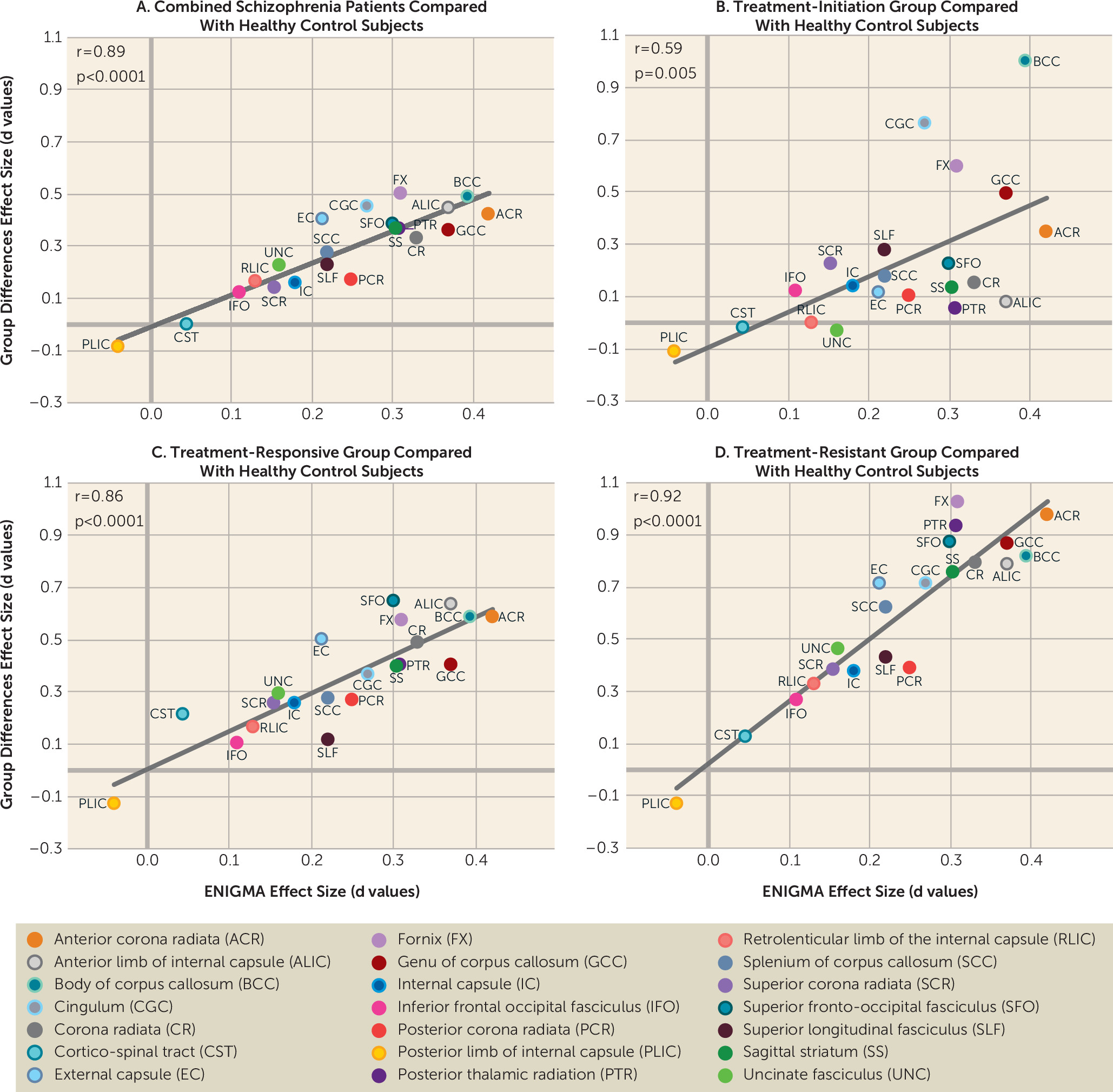
Figure 1A–
1C) and treatment resistance (
Figure 1D). Therefore, a higher RVI in the patient groups may have identified individuals with more severe patterns of neurodevelopmental white matter impairment, who in turn may be more vulnerable to treatment resistance.
Patients with treatment-resistant schizophrenia are invariably given higher doses of medications, including multiple medications, and are more likely to receive prescriptions for clozapine, compared with patients with treatment-responsive schizophrenia. These factors could confound the discovery of the neurobiology of treatment resistance by hindering the isolation of biomarkers for treatment resistance from the medication effects. To mitigate this concern, we analyzed a group of patients who were assessed within 2 weeks of initiating antipsychotic treatment. We observed a significant heterogeneity in the RVI values within this patient group. However, the candidate treatment-resistant biomarker was present in patients with minimal antipsychotic medication exposure, ruling out the possibility that higher RVI is a chronic disease effect but rather is associated with schizophrenia even at the onset of treatment. However, this study is limited by its cross-sectional nature, and follow-up studies are needed to test whether RVI observed at this stage would predict treatment resistance compared with treatment response among patients initiating treatment.
The white matter pathways most strongly associated with treatment resistance—for example, the fornix (the main white matter bundle of axonal fibers inside and outside of the hippocampus) and the anterior corona radiata (the main white matter connecting ipsilateral prefrontal cortices) (effect sizes around 1.0) (
Figure 1D)—are well-known associative pathways for supporting cognitive functions (
50,
51). In patients with treatment-responsive schizophrenia, the effect sizes of the same tracts were weaker (e.g., range for the fornix and anterior corona radiate, 0.5–0.6) (
Figure 1C), suggesting that impairments in associative tracts that support cognition may play a larger role in treatment resistance. The agreement between white matter FA regional effect sizes in patients with treatment-resistant schizophrenia in this study and patients in the ENIGMA study (r=0.92) also provides a novel perspective on the ENIGMA results. Currently available antipsychotic medications have failed to attenuate symptoms in treatment-resistant schizophrenia, having only limited effects on cognitive deficits (
52,
53). The ENIGMA-DTI sample was global, and the meta-analytical aggregation likely removed specific local medication and environmental variables, yielding schizophrenia-related neurobiology that was untreated and shared across sites. The remarkable alignment between patients in the ENIGMA study and the treatment-resistant group in this study indicates that the white matter regional effect size pattern identified in the ENIGMA study may underlie the critical unmet treatment targets (i.e., treatment resistance, in addition to cognitive deficits) (
27) in schizophrenia. It may also suggest that the white matter regional effect size pattern identified in the ENIGMA meta-analysis has limitations, because it is less strongly associated with schizophrenia during treatment initiation (r=0.58) and more strongly associated with the treatment-resistant aspect of schizophrenia.
Another potential limitation is that patients with treatment-resistant schizophrenia are treated with clozapine as the treatment of choice. Post hoc analyses did not show an association between the RVI and clozapine, and an elevated RVI was already present in the treatment-initiation group. This study focused on differences between treatment-resistant and treatment-responsive groups that were age- and sex-matched. The treatment-initiation group had a significantly lower mean age than the treatment-resistant and treatment-responsive groups, which is a potential limitation. However, the RVI was developed to be independent of age and sex, and all analyses were performed while controlling for age and sex. Finally, only FA was used to index white matter abnormalities, and other diffusion parameters (axial, radial, and mean diffusivities) were not explored. We chose FA because it showed higher sensitivity to schizophrenia deficits compared with these other parameters (
18). Future studies should reexamine these findings using more advanced DTI parameters (
54).