Baseline Frontoparietal Task-Related BOLD Activity as a Predictor of Improvement in Clinical Symptoms at 1-Year Follow-Up in Recent-Onset Psychosis
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Sample
Task Description
fMRI Scanning Parameters and Preprocessing
fMRI Analysis and Prespecified Region of Interest Selection
Linear Regression
Logistic Regression
Results
Demographic and Clinical Information
| Characteristic | Healthy Control Subjects (N=138) | Patients With Bipolar Disorder (N=17) | Patients With Schizophrenia (N=65) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Participants in the AX-1 protocol | 73 | 53 | 14 | 82 | 38 | 58 |
| Participants in the AX-2 protocol | 65 | 47 | 3 | 18 | 27 | 42 |
| Female | 53 | 38 | 7 | 41 | 16 | 25 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 20.4 | 2.7 | 21.6 | 2.8 | 20.8 | 3.3 |
| Days to follow-up | — | 429.7 | 113.0 | 384.7 | 143.7 | |
| Characteristic | Patients With Bipolar Disorder (N=17) | Patients With Schizophrenia (N=65) | All Patients (N=82) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||||||||
| BPRS scorea | |||||||||||||||
| Score improved | 8 | 47 | 39 | 60 | 47 | 57 | |||||||||
| Score did not improve | 9 | 53 | 26 | 40 | 35 | 43 | |||||||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| Antipsychotic medication | |||||||||||||||
| Received medication | 14 | 82 | 10 | 59 | 55 | 85 | 50 | 77 | 69 | 84 | 60 | 73 | |||
| Did not receive medication | 3 | 18 | 7 | 41 | 10 | 15 | 15 | 23 | 13 | 16 | 22 | 27 | |||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Chlorpromazine equivalent dosage (mg/day) | 302.3 | 156.4 | 342.5 | 358.2 | 207.2 | 148.0 | 300.7 | 298.9 | 227.4 | 154.4 | 307.3 | 305.9 | |||
Behavioral and Functional AX-CPT Results
Linear Regression
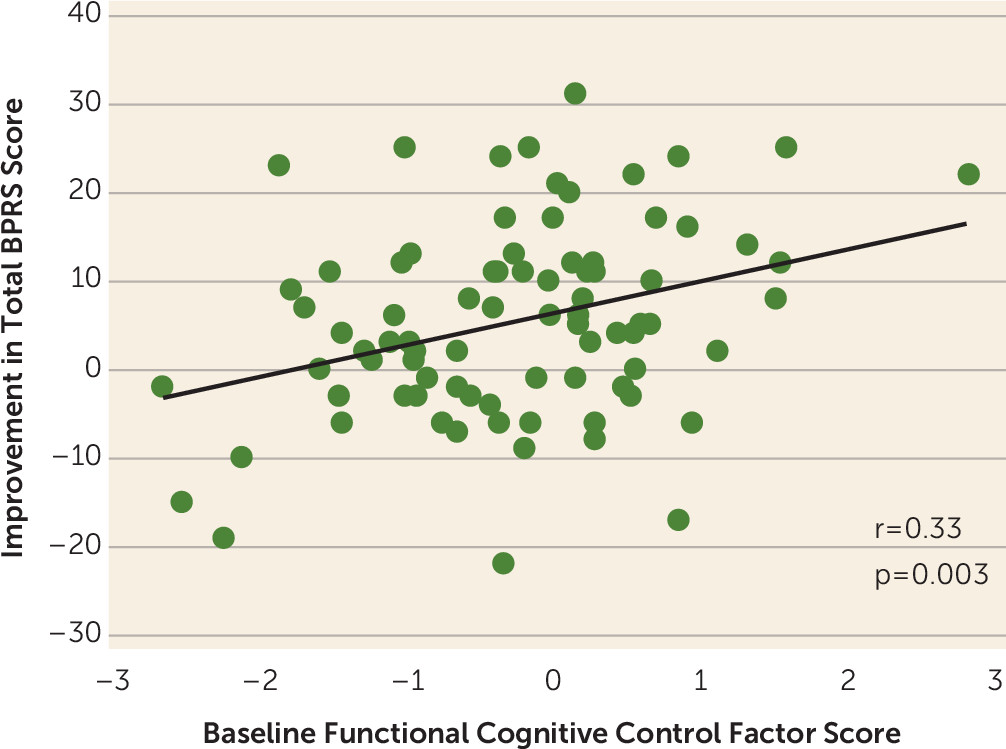
Logistic Regression
| Predictive Factor | Model χ2 | Step χ2 | Model –2LL | Model C-S R2 | Model Nagelkerke’s R2 | Predictive Factor | If Predictive Factor Removed | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p | Odds Ratio | 95% CI | Change in –2LL | p | ||||||
| Functional cognitive control factor score (DLPFC+SPC B>A cue activation) | 9.5 (p<0.01) | — | 102.4 | 0.11 | 0.15 | 0.8 | 0.3 | 0.01 | 2.2 | 1.3–3.7 | 9.5 | <0.01 |
| d′ context (adjusted z score) | –0.1 | 0.2 | 0.69 | 0.9 | 0.6–1.4 | 0.2 | 0.68 | |||||
| Constant | 0.5 | 0.3 | 0.05 | 1.7 | — | |||||||
| Functional cognitive control factor score (DLPFC+SPC B>A cue activation) | 14.0 (p=0.02) | 4.49 (p=0.21) | 97.9 | 0.16 | 0.21 | 0.8 | 0.3 | <0.01 | 2.1 | 1.2–3.8 | 8.0 | <0.01 |
| d′ context (adjusted z score) | 0.3 | 0.2 | 0.90 | 1.0 | 0.7–1.6 | 0.02 | 0.90 | |||||
| Reality distortion | 0.1 | 0.0 | 0.21 | 1.1 | 1.0–1.1 | 1.6 | 0.20 | |||||
| Disorganization | 0.1 | 0.1 | 0.40 | 1.1 | 0.9–1.3 | 0.7 | 0.40 | |||||
| Psychomotor poverty | 0.1 | 0.1 | 0.31 | 1.1 | 1.0–1.2 | 1.0 | 0.31 | |||||
| Constant | –1.3 | 1.0 | 0.18 | 0.3 | — | |||||||
| Predictive Factor | Specificity (%) | Sensitivity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Functional cognitive control factor score (DLPFC+SPC B>A cue activation); d′ context (adjusted z score); constant | 60.0 | 70.2 | 70.2 | 60.0 | 65.9 |
| Functional cognitive control factor score (DLPFC+SPC B>A cue activation); d′ context (adjusted z score); reality distortion; psychomotor poverty; disorganization; constant | 68.6 | 70.2 | 75.0 | 63.2 | 69.5 |
Discussion
Acknowledgments
Supplementary Material
- View/Download
- 197.31 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

