Major depressive disorder is the single largest contributor to disability worldwide, affecting as many as 300 million people annually (
1). Despite decades of basic science, clinical neuroscience, and psychiatric research, the pathophysiology of major depression is not well understood (
2). Neuroimaging approaches comprise a powerful, noninvasive method to investigate the neurobiological mechanisms underpinning psychiatric disorders (
3,
4). Neuroimaging’s promise notwithstanding, recent reports have challenged the reliability of this literature, drawing attention to small sample sizes (
5), clinical heterogeneity (
6), and flawed correction for multiple comparisons (
7), which jointly work to inflate false positive rates. Although improvement of neuroimaging techniques is an active area of research, previous findings are not without value. Meta-analytic approaches are capable of addressing many of the methodological concerns that contribute to varied findings at the individual study level and allow identification of reliable, true-positive findings in the literature.
Coordinate-based meta-analysis, a well-established family of methods that holds a prominent position in neuroimaging research (
8,
9), offers a large-scale data-driven approach to the identification of brain regions consistently altered by disease by testing for spatial convergence across reported findings from previously published neuroimaging studies. Coordinate-based meta-analysis tests for convergence against the null hypothesis that reported findings follow a random spatial distribution across the brain, rather than demonstrating convergent abnormality in discrete brain regions. Coordinate-based meta-analysis is applicable only to data acquired from the whole brain and analyzed in a voxel-wise manner, to ensure identification of convergent effects in a spatially unbiased (non-region-of-interest-based) manner (
8,
10). Coordinate-based meta-analysis applies equally well to multiple types of imaging data, including task activation, voxel-based morphometry (VBM) (
11), and resting-state voxel-based pathophysiology (VBP). Activation/anatomical likelihood estimation (ALE) (
10,
12–
14)—the most widely adopted coordinate-based meta-analytic method (
15)—computes the union of reported findings based entirely on location. Unlike effect-size meta-analysis, ALE is blind to magnitude and sign (+/−) of effect. Although ALE has traditionally been employed in single-modality meta-analysis, its flexibility for integration of findings across imaging methods allows for comprehensive assessment of disease-related effects. In the present study, we employed coordinate-based meta-analysis and ALE to identify convergent structural (VBM) and physiological (VBP) disease effects of both signs (+/−).
Several recent large-scale meta-analyses of structural and functional imaging in major depression and other psychiatric disorders strongly suggest that concerns about a preponderance of false positives in the neuroimaging literature are well justified.
Table 1 provides an outline of the meta-analyses described in the present summary. A transdiagnostic meta-analysis of task activation studies across several axis I disorders by Sprooten et al. (
16) found shared effects across diseases but no effect of diagnosis or Research Domain Criteria (RDoC) domain on spatial distribution of reported findings. A similar transdiagnostic ALE meta-analysis by Goodkind et al. (
17) assessed regional atrophy (VBM) patterns across six axis I disorders and did find spatial convergences in the anterior insula and anterior cingulate bilaterally when assessing across all disorders, but found no unique characteristics of any disorder. A transdiagnostic independent component analysis meta-analysis of VBM studies by Vanasse et al. (
18) assessed disease loadings on several independent brain networks. The central finding of the study was that no disease loaded on a single network, and no network loaded on a single disease. Furthermore, one of the component networks identified by Vanasse et al. closely reflected the pattern of shared pathology identified through Goodkind and colleagues’ transdiagnostic ALE analysis of VBM data. An ALE meta-analysis of resting-state VBP studies across 11 neuropsychiatric disorders by Sha et al. (
19) also identified shared effects across diseases, including widespread abnormalities in patients with major depression. However, the disease-specific distribution identified in Sha and colleagues’ analysis of major depression failed to converge at statistical thresholds recommended by ALE best-practice guidelines (
20,
21). In an ALE meta-analysis of cognitive and emotional task activation studies limited to cohorts with major depression (
6), no brain regions of significant convergence were identified. Collectively, these meta-analyses pose a challenge to the psychiatric neuroimaging community.
Findings from these transdiagnostic studies strongly indicate shared pathology across neuropsychiatric diseases and weak neurobiological “signal” of depression alone—the primary finding of the major depression–specific meta-analysis by Müller et al. (
6). Shared pathology across illnesses is “not a component of current psychiatric nosology” (
16), although these findings may align with newer research initiatives, such as the RDoC project (
22,
23). Despite the notable successes of transdiagnostic meta-analyses and the absence of significant single-diagnosis findings, disease-specific approaches remain an important area for research. The mental health care system broadly considered—care providers, insurance providers, regulatory agencies—is not likely to abandon established psychiatric terminology in light of the neurobiological observations described above. Furthermore, clinical trials testing new therapies are typically carried out in patients who fall into specific diagnostic categories, rather than being symptom-driven or transdiagnostic. For these reasons, it is important to determine whether depression-specific regional neurobiological changes can be detected using neuroimaging. Previous studies have failed to find a neurobiological “signature” of major depression alone using task activation only (
6,
16), VBM only (
17,
18), and resting-state VBP only (
19). We propose to test this hypothesis using both VBM and resting-state VBP studies in combination.
The co-localization of structural and functional abnormalities is well documented across neuropsychiatric diseases. Numerous disorders, including Parkinson’s disease (
24), Alzheimer’s disease (
25), primary progressive apraxia (
26), multiple sclerosis (
27,
28), schizophrenia (
29), and mood disorders, including major depressive disorder and bipolar disorder (
30,
31), have demonstrated conjoint abnormalities of brain function and structure, and recent research has investigated this relationship in major depression specifically (
32,
33). The concordance of structural and functional abnormalities in neurodegenerative diseases underlies the network degeneration hypothesis (
34–
36), for which major depression is also being investigated as a potentially network-based disorder (
37–
40). Furthermore, recent research investigating the network degeneration hypothesis indicates that high-traffic network “hubs” are more likely to experience gray matter lesions as a result of disease-related overstimulation (
41,
42), which may contribute to subsequent decreases in brain function in affected regions. As we anticipate the co-localization of structural and functional disease effects in major depression, a central objective of the present study was to examine the convergence of gray matter atrophy and both increases and decreases in resting-state function, both independently and jointly. Currently, VBM investigations of gray matter alterations in major depression form a large body of literature suitable for meta-analysis. Similarly, VBP investigations using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging of brain blood flow and glucose metabolism, together with recent advances in functional MRI (fMRI) techniques, contribute to a growing VBP literature in major depression. The present study is among the first to comprehensively assess resting-state functional (VBP) and structural (VBM) findings in major depression both independently and in pooled multimodal data sets.
Thus, our objective in this meta-analysis was to assess the spatial convergence of brain abnormalities in major depression as detected by structural and resting-state functional neuroimaging data. Our primary hypothesis was that major depression would demonstrate pathological changes detectable across neuroimaging paradigms. We hypothesized localized convergence of gray matter atrophy and increased and decreased brain function in patients with major depression relative to control subjects. We also hypothesized improved co-localization of abnormalities, as evaluated through pooled data sets for secondary analysis. The meta-analytic design and statistical thresholds for this study were selected to emulate Müller and colleagues’ 2017 study (
6) in order to compare the findings from task-based and task-independent investigations of major depression. We hypothesized further that accounting for the clinical heterogeneity of major depression, by assembling patient subgroups (to the degree possible through the available literature), could enhance the convergence of identified brain regions. Hypotheses confirmed by this meta-analytic approach, we would submit, should be regarded as providing direction for further primary data studies, rather than seen as established conclusions.
Methods
Literature Search
A search of PubMed, Google Scholar, and BrainMap (
18,
43–
45), as well as reference tracing of previous meta-analyses, was performed to identify neuroimaging experiments in major depression reporting gray matter atrophy, increased resting-state function, or decreased resting-state function compared with healthy control subjects. Major depression–related hypertrophy, a rare phenomenon occasionally reported in remitted major depression (relative to acute major depression), was not included in this analysis. VBM studies and resting-state VBP studies of regional cerebral blood flow, regional homogeneity, amplitude of low-frequency fluctuations (ALFF) and fractional ALFF (fALFF), and regional glucose metabolism were identified using various combinations of the search terms
major depressive disorder,
major depression,
depression,
unipolar depression,
VBM,
gray matter,
regional cerebral blood flow,
PET,
SPECT,
arterial spin labeling,
regional homogeneity,
ALFF/fALFF,
glucose metabolism,
brain activity, and
resting state. The literature search was completed in January 2018.
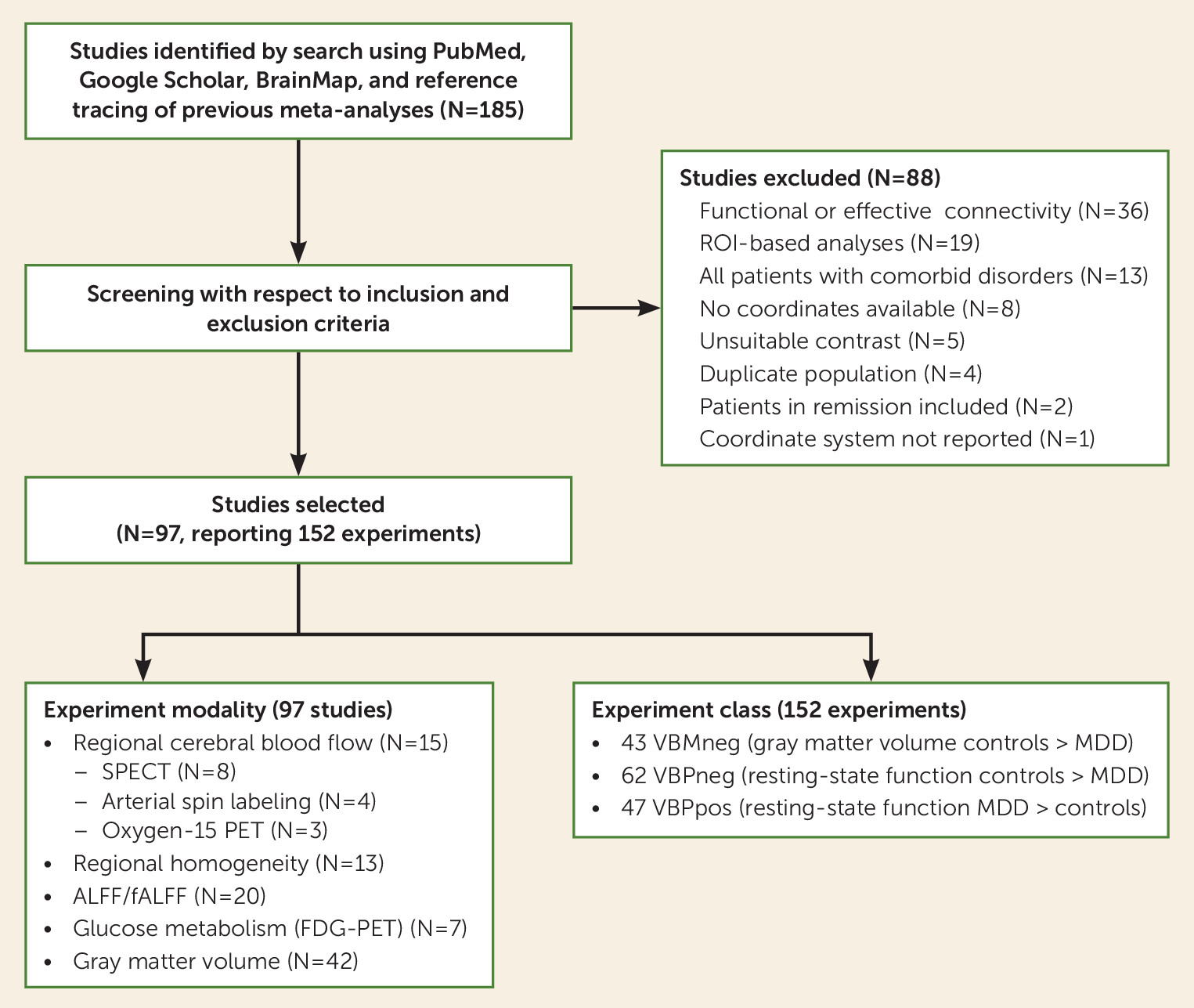
Figure 1 is a study selection diagram for this meta-analysis (further details of the literature search are provided in Appendix 1 in the
online supplement).
Study Selection Criteria Relating to Indices of Quality
Preliminary selection criteria required that studies be peer-reviewed English-language neuroimaging reports. Studies identified outside the BrainMap database were reviewed by BrainMap team members and subsequently coded through Scribe and submitted to the database (
21). Standard expectations for publication in this field is for application of motion correction, measures to limit motion during scan, and/or exclusion of data that exhibited excessive motion during acquisition. Measures for motion correction utilized in each included study are included in Table S1 in the
online supplement. Studies that did not specify measures for motion correction were not excluded on that basis alone.
Study Selection Criteria Relating to Subjects
Patients with major depressive disorder from included studies were diagnosed using DSM-III (four studies), DSM-IV (85 studies), or ICD-10 (three studies) criteria. Only studies comparing patients in the acute phase of major depression to healthy control subjects were included. Experiments including remitted subjects (N=2) or any contrast other than major depression versus healthy control subjects were excluded (N=5) (see
Figure 1). Studies utilizing dual-diagnosis patient populations with other major medical illness (e.g., major depression and hypothyroidism) or psychiatric comorbidities were excluded (N=13). However, we allowed for the inclusion of studies in which partial populations of the patient cohort had comorbidities (e.g., a subset of depression patients with anxiety symptoms) as long as major depressive disorder was the primary diagnosis. Studies with strict exclusion criteria for psychiatric comorbidities were flagged for use in subsequent meta-analytic grouping. We allowed for the inclusion of studies that featured patient populations of varying medication status, but flagged studies that recruited patient populations of specific medication status (all medicated, treatment naive, drug washout) for subsequent meta-analytic grouping. We also flagged studies that recruited patient populations of specific severity or disease onset (first episode, chronic/recurrent, treatment resistant, adolescent, geriatric).
Study Selection Criteria Relating to Technical Aspects
Studies of resting-state VBP included investigations of regional cerebral blood flow, regional cerebral glucose metabolism, regional homogeneity, and ALFF/fALFF using PET, SPECT, and fMRI. Included resting-state VBP studies had to have used voxel-wise whole-brain methods to compare patients with major depression to healthy control subjects. Thirty-six studies investigating functional or effective connectivity were excluded from this meta-analysis because they used regional sampling (N=26), used incompatible patient-group contrasts (N=2), were review articles or meta-analyses (N=4), or were multivariate analyses only, without mass-univariate analyses (N=2), and the remaining studies (N=2) were excluded because as functional or effective connectivity studies they cannot be integrated in current coordinate-based meta-analysis methods.
Included studies of gray matter volume utilized VBM methods. Included studies had to have used voxel-wise whole-brain methods to compare patients with major depression to healthy control subjects. Studies using non-whole-brain methods, such as region-of-interest or network-restricted sampling (N=19), were excluded.
Only studies reporting results as coordinates using standard reference space (Talairach or Montreal Neurological Institute) were included; studies that did not report results in the form of standardized coordinates (N=8) or did not report the coordinate system used (N=1) were excluded. Coordinates were converted to Talairach space for this analysis (
46). To avoid repeated inclusion of the same patient populations, we carefully screened studies that drew from open-source or national data repositories and excluded those that reported use of a patient cohort already included in this meta-analysis (N=4).
Data nonredundancy is of crucial importance to avoid bias in meta-analytic findings. To avoid inclusion of duplicate patient populations, we performed several pre and post hoc assessments. First, we carefully screened studies that drew from open-source or national data repositories and excluded those that reported duplicate patient cohorts (N=4). For multiple studies deriving from the same research group, patient populations and reported coordinates were inspected to assess potential redundancy (
18). For studies reporting multiple contrasts from the same patient population, only one contrast per patient population was used in each meta-analysis (
47). Post hoc assessments were performed in cases where multiple experiments from the same research group contributed to identified clusters. In these cases, leave-one-out analyses were performed to assess potential redundant contributions to identified clusters. In cases where potential patient overlap was indicated by leave-one-out analysis, only the largest study was included for final meta-analysis.
All-Effects Analysis
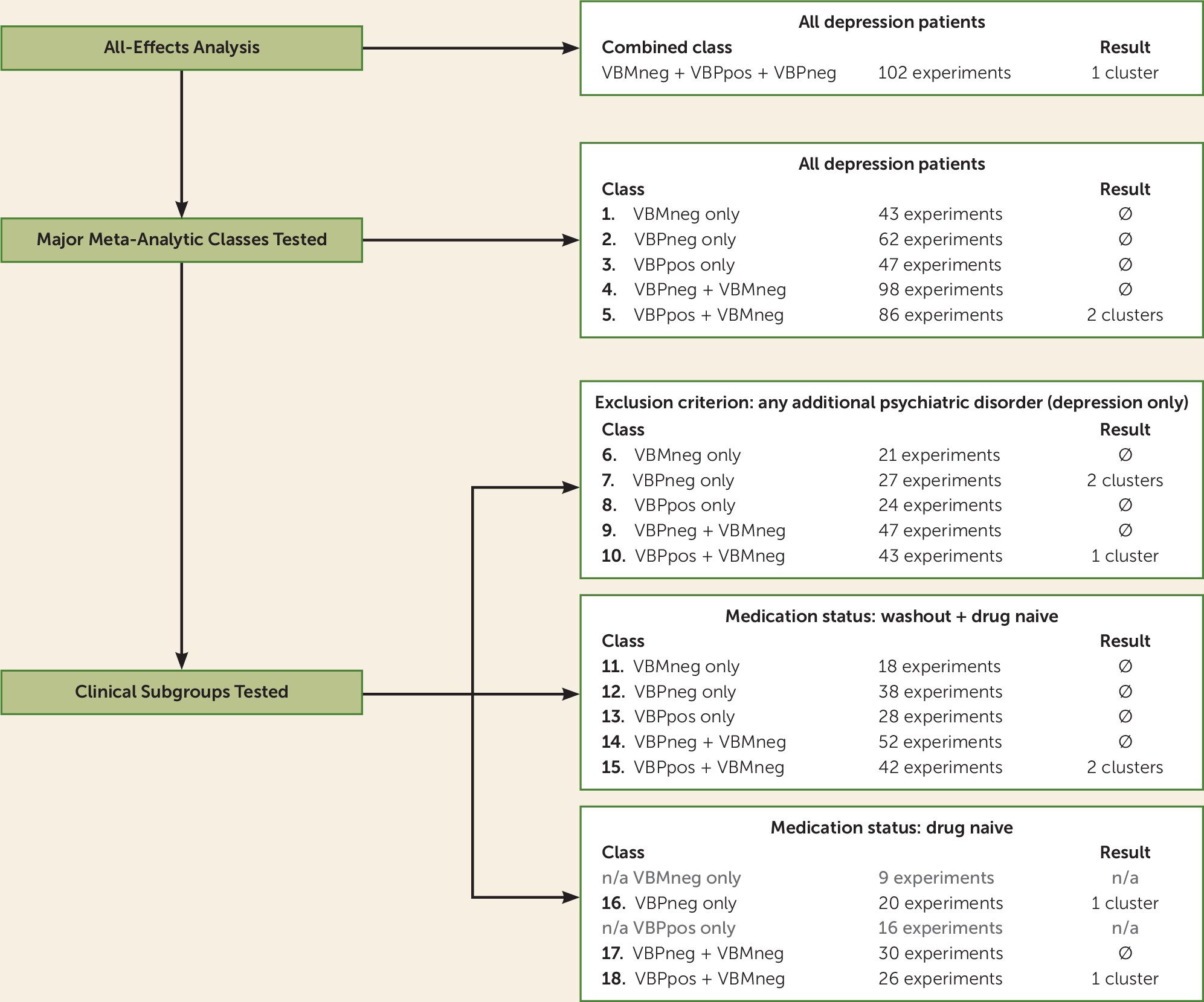
Coordinates from all included studies were collectively pooled to generate a unified all-effects meta-analytic category. Coordinates from multiple experimental contrasts obtained from the same subject group (such as studies that reported both locations of gray matter atrophy and locations of increased or decreased function relative to control subjects in separate experiments) were concatenated to generate sign-independent foci groups for each patient population tested. (See Appendix 2 in the online supplement for details of ALE analysis.) All patients, including both medicated and unmedicated patients at time of scanning, were included in the all-effects analysis.
Meta-Analytic Data Classes
To assess modality-specific contributions to findings from the all-effects analysis, input data were grouped into primary and secondary meta-analytic groups for analysis. Primary analyses included three single-modality classes and two dual-modality classes, as follows. Three single-modality classes were created by grouping experiments of decreased gray matter volume in patients with major depression compared with control subjects (VBMneg), decrease in resting-state function in patients with major depression compared with control subjects (VBPneg), and increased function in patients with major depression compared with control subjects (VBPpos) (
Figure 2, items 1, 2, and 3); two dual-modality classes were created by combining the classes of VBPneg and VBPpos with the gray matter volume data (VBPneg+VBMneg and VBPpos+VBMneg) (
Figure 2, items 4 and 5). In studies that reported both VBM and VBP changes in the same subject population, we included only the coordinates reporting change in VBP in the pooled data sets (N=5). For initial analysis of the five major meta-analytic classes, all available data (including patients of varying medication status at time of scan) were included to test convergence of clinically heterogeneous patient groups.
Patient Groupings
Studies were grouped for analysis into two tiers: all-effects (all patient types) and patient subgroups. Studies were subgrouped as 1) drug/treatment-naive major depression only, 2) treated major depression with drug washout before imaging, and 3) major depression with no psychiatric comorbidities. For each subgroup, all five classes of experiments were analyzed, provided the number of included experiments was sufficient for robust ALE calculation (
Figure 2, items 6 through 18). Per Eickhoff et al. (
20), the minimum number of experiments required for robust ALE analysis is 17.
Other subgroups attempted (first episode, chronic/recurrent, treatment resistant, adolescent, geriatric) did not include a sufficient number of individual experiments for meta-analysis (see the Discussion section). Drug-washout groups also did not include a sufficient number of experiments for stand-alone analysis. In an effort to maximize the use of available information from individual studies, we combined the drug-washout and drug-naive groups to assess potential effects from a medication-free group.
ALE Meta-Analysis
Activation likelihood estimation (ALE) (
8,
10,
14,
20) was performed using GingerALE, version 3.0 (
48). The ALE algorithm was originally developed for use in task activation functional studies (
12) but has undergone numerous revisions, including adaptations for use with VBM studies (
47,
49). ALE assesses spatial convergence of reported findings against the null hypothesis that findings follow a random spatial distribution rather than demonstrating statistically significant convergence at discrete regions. The most current versions of the ALE model reported coordinates, or foci, as three-dimensional Gaussian probability distributions to generate per-experiment modeled activation (or modeled atrophy) maps (
50). ALE derives full-width half-maximum for each Gaussian distribution based on sample size, allowing experiments with larger samples greater statistical certainty. ALE generates a union map of all per-experiment modeled activation maps and tests for above-chance spatial convergence through a variety of available thresholding options. A revised version of the algorithm (
48) recommends either family-wise error or cluster-level inference thresholding methods for robust analysis. The selected method for the present study, cluster-level inference, generates a simulated data set of randomly distributed foci based on characteristics of the input data set for testing the null hypothesis.
Results were thresholded for significance using cluster-level inference of p<0.05 with a cluster-forming threshold of p<0.001 to reflect the study design from Müller and colleagues’ 2017 study (
6) and ALE best practices (
21,
51). ALE analysis was retested at cluster-level inference of p<0.0027 (Bonferroni correction for multiple comparisons of 18 total meta-analyses) to assess the robustness of identified clusters.
Noise Simulation for Estimation of File-Drawer Effect
The potential for unpublished null findings in the neuroimaging literature is not currently accounted for in the ALE algorithm, as ALE’s focus is to assess convergence of nonnull findings, of which a large portion are anticipated to be false positives (
52). Potential publication bias in the present study was evaluated through a modified version of the fail-safe N method described by Acar et al. (
15) to estimate the robustness of identified results against unpublished neuroimaging findings. A recent simulation utilizing the BrainMap database determined that the rate of missing contrasts may be estimated at 6 per 100 instances of reported findings (
53). Thus, we retested convergent meta-analyses with an additional 6% added noise to assess the robustness of identified clusters. Surviving clusters were subsequently retested with higher rates of noise up to 30%.
Results
A total of 92 articles (97 studies) with 152 individual experiments comprising results from 2,928 patients were identified for inclusion in this meta-analysis. The number of experiments included in each major meta-analytic category were as follows: VBMneg, 43 experiments; VBPneg, 62 experiments; and VBPpos, 47 experiments. Tables S1 and S2 in the online supplement list all studies included in meta-analysis, and Figure S1 shows the distribution of foci from each of the major meta-analytic categories.
All-Effects Analysis
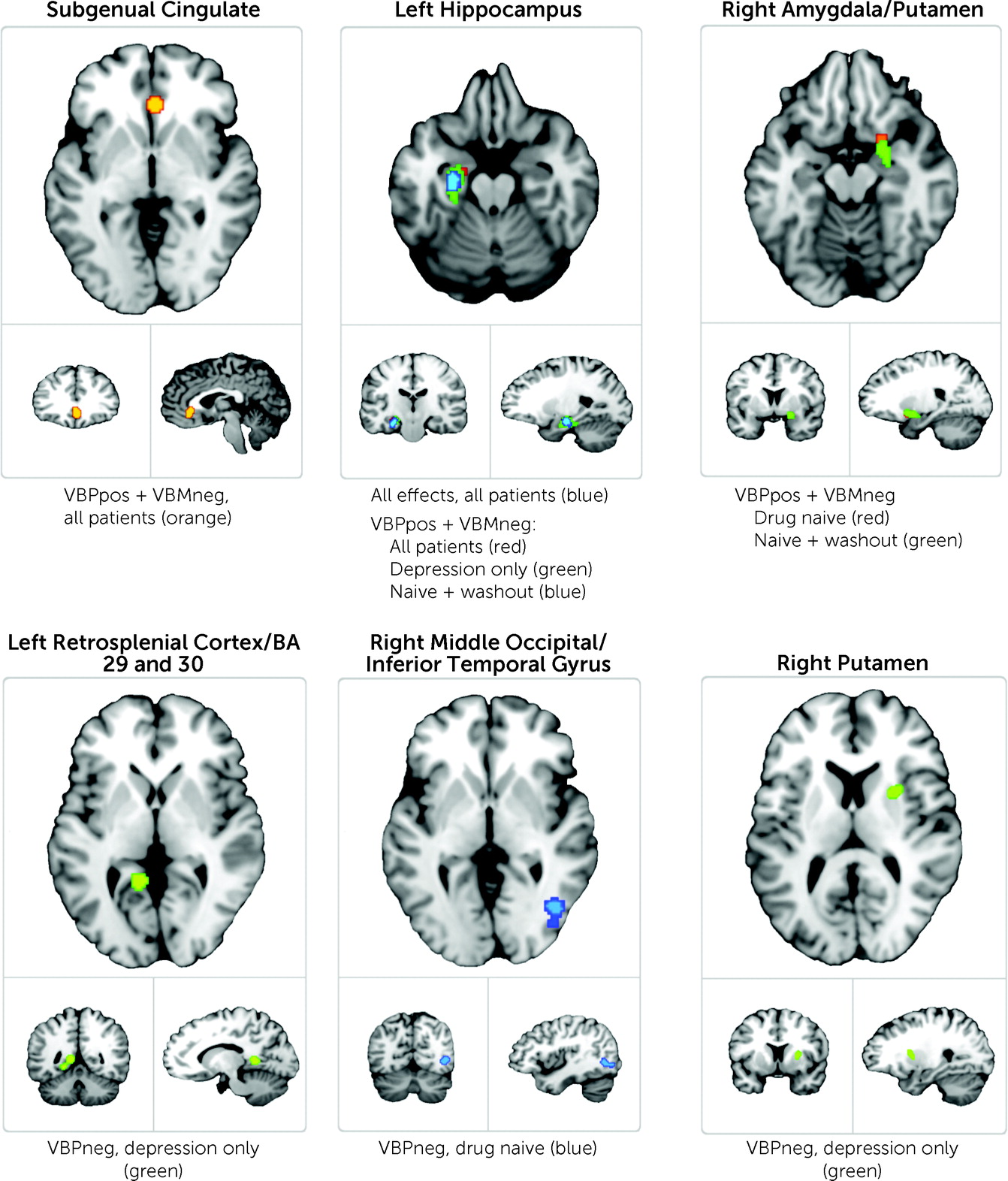
The all-effects analysis comprised a total of 102 foci groups created from summed results from all experiment types. This unified analysis identified a single region within the left hippocampus as demonstrating convergent abnormality, as listed in
Table 2 and shown in
Figure 3.
Heterogeneous Group: All Patients
Among the classes of gray matter volume, VBPneg, VBPpos, and VBPneg+VBMneg utilizing all pooled patient data, none revealed any significant regions of convergent brain abnormality. The class of VBPpos+VBMneg utilizing clinically heterogeneous patient data identified consistent aberrant brain regions in major depression within the left hippocampus (as identified in the all-effects analysis) and an additional region of significant convergence in the subgenual cingulate cortex (see
Table 2 and
Figure 3).
Patient Subgroups
Clinical subgroups that fulfilled the criterion of N>17 experiments included the categories of drug-naive patients, drug-naive and drug-washout patients combined (naive+washout), and those studies with strict exclusion criteria for comorbid psychiatric disorders for patients with major depression (depression-only). (See Tables S3–S8 in the online supplement for lists of studies included in each meta-analytic grouping.)
A total of 13 clinical subgroups across the five meta-analytic classes included a sufficient number of experiments to perform ALE analysis. Among the 13 subgroups, only five yielded significant results (see
Table 2 and
Figure 3). Clinical subgroups within the VBPpos+VBMneg class identified significant convergence among the drug-naive, naive+washout, and depression-only clinical groups. Consistent abnormal brain areas identified within these clinical subgroups included regions of the left hippocampus (as previously identified) and an additional region including areas of the right amygdala and ventral anterior putamen. Clinical subgroups from the VBPneg class identified significant regions among the drug-naive and depression-only clinical groups. Areas identified within these clinical subgroups included three regions in addition to those previously identified: a region encompassing areas of the left middle occipital and left inferior temporal gyri, a region within the left retrosplenial cortex, and a region within the right putamen.
Convergence by Imaging Modality
Data from various imaging modalities contributed to the clusters identified through ALE analysis, with no single modality being profoundly overrepresented in any result from the VBPpos+VBMneg class. Regions identified in clinical subgroups of the VBPneg class were somewhat dominated by contributions from ALFF and regional homogeneity experiments, although this could be attributed to their overall representation within the data set tested. (For detailed distributions, see Tables S9–S11 in the online supplement.)
Noise Simulation
Each identified cluster was retested in meta-analyses with added noise (beginning at 6% noise) to assess robustness against potentially unpublished findings. Surviving clusters were subsequently retested with higher rates of noise up to 30%.
Table 2 details the fail-safe N percentage of additional noise that must be added to each meta-analysis to result in failure of convergence for previously identified clusters. In general, noisier contrasts (e.g., all-effects and other groups including all patients) are less robust against the simulation of additional noise.
Discussion
Our findings do not support our first three hypotheses, with no brain regions of significant convergence arising from meta-analysis of gray matter atrophy or increased or decreased brain function in patients with major depression compared with controls. However, brain regions demonstrating significant abnormality did arise from meta-analysis of pooled structure-function findings in major depression, supporting our hypothesis of co-localized effects. We also identified additional regions of convergence from meta-analysis of clinical subgroups despite decreased sample size.
The present work, to our knowledge, is the first to comprehensively assess multimodal imaging data to investigate the convergence of VBM and VBP findings in major depression. Regions of significant convergence identified in the study include the subgenual cingulate cortex, the left hippocampus, the right amygdala/putamen, the left retrosplenial cortex, and the right middle occipital/inferior temporal gyri. The brain regions identified in our meta-analysis are included in many current models of depression pathology and treatment approaches. Furthermore, our methods of largely pooling multimodal data and, conversely, delineating data by available clinical details, improved convergence of results. Our identification of brain regions demonstrating reliable abnormality in major depression—whereas previous meta-analyses have failed to identify any disease-specific effects in major depression—is a significant contribution to the literature. We view these results as a motivation for refinement of future primary studies in major depression.
Identified Regions
Identification of consistent abnormality within the subgenual anterior cingulate cortex in this meta-analysis is a potentially important finding for depression research. The subgenual cingulate has been widely implicated in major depression as a regulator of mood (
30,
31,
54–
56), in the processing of emotional stimuli (
57–
59), and as a target for network-based treatments such as deep brain stimulation (
60–
63) and a downstream target for transcranial magnetic stimulation (
64–
67). Reliable identification of the subgenual cingulate through large-scale multimodal meta-analysis strongly supports further research on this region’s role in major depression. Furthermore, the subgenual cingulate has not been reliably identified in transdiagnostic meta-analyses, such as the VBM-ALE analysis conducted by Goodkind et al. (
17) (although the subgenual cingulate does appear to be present in the all-groups analysis; see
Figure 2A). This distinctive finding arising from the present study suggests that disease-specific effects, beyond transdiagnostic-only effects, are detectable in neuroimaging data and warrant further exploration.
The left hippocampus was also identified in this study. Decreased hippocampal volume has been observed in neuroimaging studies of major depression over the past 20 years (
68–
72). Hypotheses of major depression–related decline in hippocampal volume posit that the hippocampus may be affected by stress (
72) and may contribute to the cognitive (
73) and recollection memory deficiencies (
74,
75) often present in individuals with major depression. The hippocampus has also been implicated in major depression through disrupted hippocampal connectivity effects on self-referential activity in major depression (
76), and conjoint reductions in gray matter density and activation during working memory tasks have been demonstrated in patients with major depression (
77). The identification of the hippocampus in the present study is a notable finding for both past and future investigations of this brain region’s role in major depression.
We also identified regions of the right amygdala and right putamen in this study. In recent investigations utilizing emotional valence paradigms, the amygdala has demonstrated aberrant activation in patients with major depression compared with healthy control subjects (
78). The amygdala was also found to demonstrate reliable volume differences in unmedicated patients with major depression relative to control subjects in a meta-analysis of 13 individual neuroimaging studies (
79). The putamen, although its potential role is less well established in major depression, has also demonstrated volumetric and shape abnormalities in untreated first-episode major depression (
80). It has also demonstrated functional disruption in major depression through investigation of the correlation between anhedonia severity and aberrant neural activity in response to emotional stimuli (
81). Our findings suggest the need for continued investigation of the potential roles of the amygdala and putamen in major depression.
Other regions identified in this study, including the left retrosplenial cortex (encompassing regions within Brodmann’s areas 29 and 30) and an overlapping area of the right middle occipital and inferior temporal gyri, do not have well-established roles in current models of major depression. A recent large study (
82) of patients with major depression (N=336) demonstrated that altered functional connectivity between the retrosplenial cortex and other key brain regions may contribute to increased rumination symptoms in depression. Brain perfusion deficits in occipital areas have been observed in adolescents with major depression (
83), although that study provided uncertain conclusions for the region’s significance in major depression. More recently, increased functional connectivity with the right middle occipital gyrus and the amygdala has been observed in association with cognitive dysfunction in major depression (
84). The right middle occipital and inferior temporal gyri also demonstrated reduced cortical thickness in patients with major depression compared with control subjects in a recent large-scale study from the ENIGMA cohort (N=1,902) (
85). Although the role of these regions in major depression is less well defined, results from the present study indicate that further investigation of these regions’ potential role in the pathophysiology of major depression is warranted.
Convergence From Patient Groupings
Separation of data into patient subgroups played a critical role in identifying additional brain regions beyond those found in the more heterogeneous groups. To our knowledge, this is the largest meta-analysis in major depression that also included a sufficient number of experiments to perform ALE analysis in subgroups. Our findings suggest that clinical heterogeneity in major depression has observable neuroimaging effects, which warrant further investigation.
Our study was limited by the recruiting and reporting methods employed at the individual study level. Author-defined patient groups in the present study were largely limited to medication status (treatment-naive and drug-washout groups). Other categories, including patient groups of specific severity (first episode, recurrent/chronic, treatment resistant) and age at onset (geriatric or adolescent depression) did not meet the N>17 experiments criterion (
20) and were not further analyzed in our study. Of note, 60% of the experiments included in the drug-naive categories (VBPpos+VBMneg and VBPneg drug-naive groups) included experiments reporting findings from patients with first-episode major depression (see Tables S5 and S8 in the
online supplement). Findings from these subgroups may indicate neuroimaging effects specific to first-episode major depression, although we were not able to reliably test this effect in the present analysis. Thus, we strongly recommend that this potential effect be investigated in future studies.
More meaningful patient categories for future studies would ideally focus on severity, duration, and treatment response in major depression. Of the 97 studies included in this analysis, 42 studies (43% of the total) recruited mixed major depression patient populations and pooled all patients into heterogeneous groups regardless of age at onset, disease duration or severity, and number of previous episodes. It is a common convention in neuroimaging publications to include patient demographic tables reporting the mean and standard deviation of these clinical features, although this is not standardized or consistent in the literature. A central recommendation from the present work is for standardized recruiting and reporting mechanisms to be adopted at the individual study level. Given the heterogeneous presentation of major depression, investigations of more homogeneous patient populations would both improve the interpretation of findings at the individual study level and promote more meaningful investigations at the meta-analytic level.
Convergence From Imaging Modalities
A central finding from this study is the failure of convergence in the three single-modality meta-analyses and successful convergence—albeit relatively weak—in pooled multimodality data sets. To our knowledge, this is the largest meta-analysis in major depression to date and the first to pool results across imaging modalities (VBM and VBP) in this manner. Identification of convergent brain abnormalities across structural and functional data sets supports our hypothesis for the co-localization of disease effects in major depression. This co-localization, and/or longitudinal progression, of major depression–specific disease effects is not well established in the literature and calls for further investigation. As the meta-analyses here were greatly facilitated by access to the collated and coded VBM literature shared in the BrainMap database, we anticipate that expanding BrainMap to include a sector sharing the resting-state VBP literature will be an important tool for future meta-analyses.
Overall, convergent findings in the present meta-analysis are sparse compared with the volume of input data (see Figure S1 in the
online supplement). Although the identification of regions of significant convergence in this study is a distinct advance from previous meta-analyses—which failed to yield any convergent findings—the sparseness of our results is nonetheless notable. In our largest analysis (all-effects) only eight of 102 experiments contributed to the identified region of convergence. In our smallest analysis (VBPneg, drug-naive) only three of 20 experiments contributed to the identified region (see Table S12 in the
online supplement for details). The small number of contributing experiments is a stark contrast to the large volume of findings reported at the individual study level. The sparse findings from this study may be attributable to our analysis of exclusively voxel-wise whole-brain studies. Sprooten and colleagues, in a 2017 meta-analysis (
16) (which included analysis of both whole-brain and region-of-interest-based studies), found that although region-of-interest-based approaches seem more adept at yielding significant findings, this may be due to confirmation bias stemming from a priori selection of brain regions for selective analysis. Sprooten et al. reported that as a result of this bias, there may be an artificial exaggeration of particular brain regions’ role in psychiatric diseases, and findings from region-of-interest-based studies should therefore be interpreted with caution. Considering the broad neuroimaging literature, the scarcity of results at the meta-analytic level underscores the impact of region-of-interest-driven findings (
16), clinical heterogeneity (
6), and the overall replication problem in the current literature.
Finally, our findings suggest the possibility of improved convergence in task-independent data compared with task activation data. The lack of convergence in the previously reported task activation meta-analysis by our group (
6) was speculated to be due in part to confounders introduced through inconsistency of the processes investigated in various tasks. Further pitfalls of task-activation-based studies include the dependence of task-based paradigms on patient cooperation (
86) and the lack of diagnostic specificity in findings from task-based fMRI studies (
16). The advantages of task-free paradigms, however, especially for use in meta-analysis, are not definitively addressed in the literature and warrant further investigation.
Limitations
A primary motivation for this meta-analysis was to compare task-independent findings to those from the 2017 Müller et al. study (
6), which utilized task activation data. Thus, after the all-effects analysis, we performed post hoc analysis (without correction for multiple comparison) utilizing the same parameters from the earlier study for significance threshold and design for sub-meta-analyses (cluster-level inference of p<0.05 with a cluster-forming threshold of p<0.001). We have also reported the clusters that prevail after Bonferroni correction (cluster-level inference restricted to p<0.0027) in
Table 2. The survival of two regions identified from the drug-naive and depression-only subgroups further supports our conclusion that sample homogeneity in major depression plays a major role in convergence of neuroimaging findings.
Another limitation of this study is the inability of current methods for coordinate-based meta-analysis to integrate findings from functional and effective connectivity studies. Functional and effective connectivity studies represent a rich corpus of work: 36 were identified in the literature search for the present study. However, 26 of these were regionally restricted, using regions of interest for analysis or seeding, making them ineligible for ALE coordinate-based meta-analysis. Only two resting-state functional connectivity studies were whole-brain and voxel-wise studies. These were not included in the present analysis.
Finally, as previously discussed, recruitment of clinically heterogeneous samples of patients with major depression at the individual study level substantially contributed to the limitations of this study. Whole-group analyses were limited by varied medication status and other factors relating to clinical heterogeneity of major depression, which we tested for in subgroup meta-analyses to the best of our ability. Forty-three percent of the studies we included in this analysis recruited mixed samples of patients with major depression and pooled all patients into heterogeneous groups regardless of age at onset, disease duration or severity, and number of previous episodes. As discussed in the Methods and Results sections, the testing of subgroups based on depression severity (first episode, chronic/recurrent, treatment resistant) would have provided more clinically meaningful findings. Thus, our findings from clinical subgroups limited to medication status may not definitively indicate neurobiologically homogeneous patient characteristics and could instead be due to other methodological factors that we were not able to test for reliably. However, delineating patient groups to the best of our ability did improve convergence of results and indicates that clinical heterogeneity of major depression warrants further investigation in neuroimaging studies.
Conclusions
The study findings suggest that major depressive disorder exhibits a concordance of abnormality in both structure (VBM) and function (VBP) in selected brain regions. Our findings suggest the presence of major depression–associated brain features, in contrast to lack of disease-specific findings from previous transdiagnostic and major depression–specific meta-analyses. Per our successful integration of VBP findings, we recommend the addition of a VBP sector to the BrainMap database to facilitate future meta-analyses in this area of study. Finally, our analysis of clinical heterogeneity within this meta-analysis suggests that diverse patient populations may present significant confounders in neuroimaging findings in major depression.