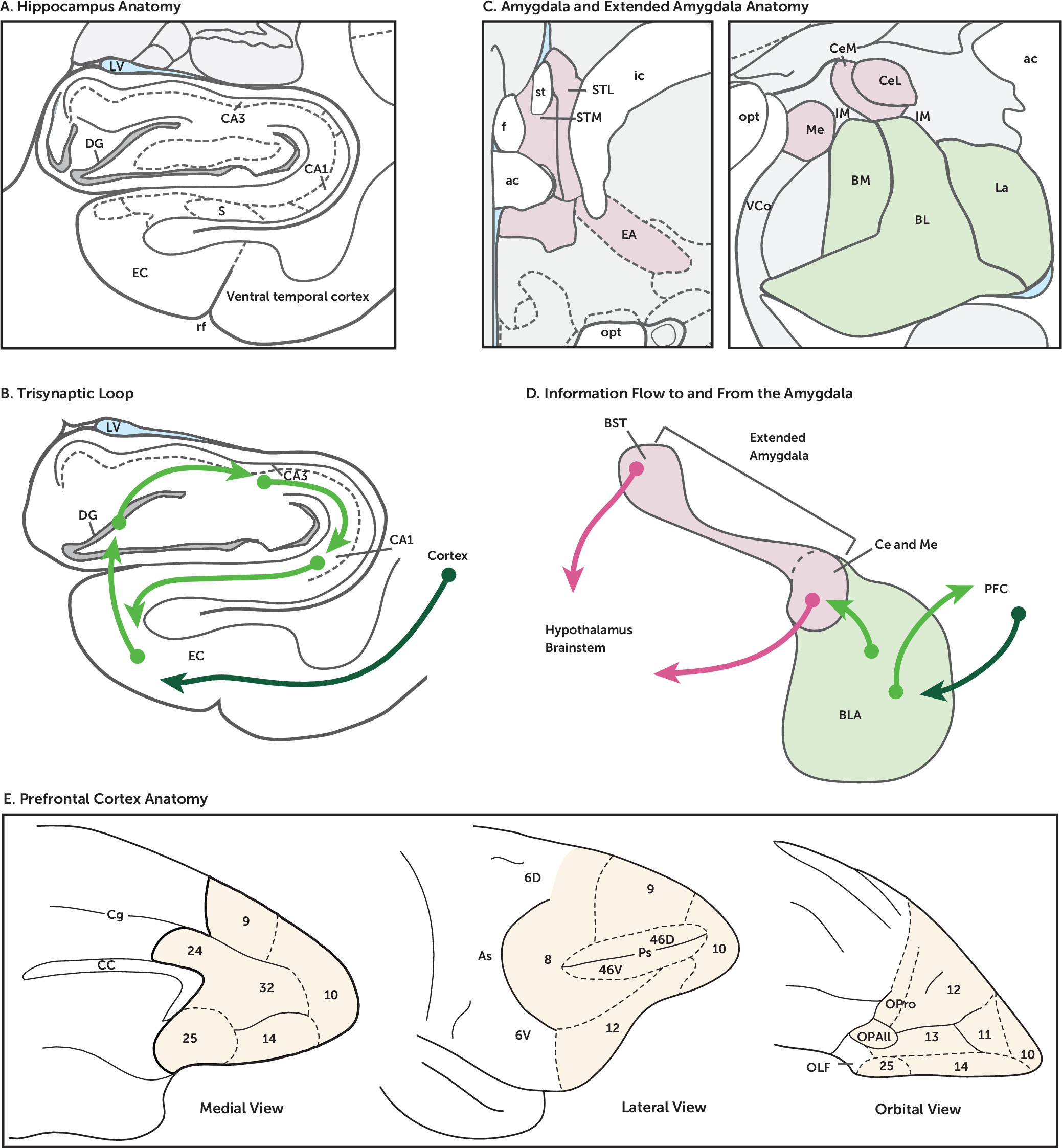
The evolutionarily conserved “limbic system” is a set of brain structures involved in emotion, motivation, learning, and memory (
4). The exact brain regions that comprise the limbic system have been redefined as we learn more about the structure, function, and connectivity of each region, but at its core is the medial temporal lobe (MTL) (
5). The MTL is composed of the cerebral cortices on the ventral and medial surface of the anterior temporal cortex, as well as the gray matter structures that lie deep beneath the cortical surface. “Medial temporal lobe” is a term typically associated with the tissues surrounding the rhinal fissure, known as the parahippocampal region (perirhinal, entorhinal, and parahippocampal cortices), along with the subcortical hippocampus, a set of highly interconnected neuronal sheets (dentate gyrus, CA fields, and subiculum) critical for the formation of long-term memory (
Figure 1A,B). While the hippocampus has been traditionally associated with the MTL, the amygdala, a grouping of nuclei situated immediately anterior to the hippocampus, also constitutes part of the MTL (
Figure 1C,D).
In addition to the amygdala and hippocampus, typically included in the definition of the limbic system are the cingulate gyrus, anterior thalamic nuclei, septum, mammillary bodies, and associated white matter tracts (e.g., fornix, stria terminalis). The classic concept of the limbic system was born out of the early work of MacLean, who introduced the concept of the “triune brain” (
6). This concept was based on evidence from comparative anatomy, neurochemistry, and behavior, positing that the complexity of the mammalian brain was built by evolution in stages corresponding to three systems: protoreptilian, paleomammalian, and neomammalian (
7). With reference to the observations and nomenclature of the anatomist Broca (
8), MacLean referred to the paleomammalian brain as limbic (from the Latin
limbus, meaning border), because its structures form a border between the evolutionarily recent neocortex and the primitive structures of the brainstem. These limbic structures overlapped with the Papez circuit, which was hypothesized to underlie the expression of emotion in animals (
9). Subsequent work by Klüver and Bucy demonstrated that the structures of the MTL were important for emotion-related behaviors, as removal of the anterior MTL in monkeys led to a flattening of affect or “tameness” and indiscriminate oral and sexual behaviors (
10,
11).
The prefrontal cortex (PFC) is a large area of the cortical mantle covering the anterior portions of the frontal lobe (
Figure 1E). Regions of the PFC are bidirectionally connected to the limbic system and via these pathways are thought to coordinate and regulate cognitive-emotional processes (
12). These higher-order cognitive functions found in nonhuman primates and humans are consistent with the PFC’s later evolutionary development. The most striking evolutionarily related anatomical developments of the PFC include its laminar structure and interconnectivity within and among its numerous divisions (
13). It is this complexity that is thought to underlie the abstract thought, emotions, and behaviors that are unique to primates and that also makes them vulnerable to developing maladaptive responses and psychopathology (
14,
15). A number of white matter tracts, including the uncinate fasciculus, superior longitudinal fasciculus, fronto-occipital fasciculus, and ventral amygdalofugal pathway, carry neuronal fibers connecting the PFC with the MTL and other regions of the cortex. Below we provide more detail regarding the anatomy and function of the hippocampus, amygdala, and PFC that is relevant to understanding pathophysiological processes underlying psychopathology.
Hippocampal Anatomy, Function, and Dysfunction
The microcircuitry of the hippocampus contains one of the most well-known and well-studied set of synaptic connections. Known as the trisynaptic loop (
Figure 1A-B; dentate gyrus-CA3-CA1) and made famous by the iconic drawings of Ramón y Cajal, this arrangement exists along the entire long axis (anterior-posterior in primates, dorsal-ventral in rodents) of the hippocampus. It is this pronounced, unidirectional laminar circuit that allowed for the initial discovery of long-term potentiation, the electrophysiological phenomenon of synaptic plasticity and the biological instantiation of Hebb’s famous hypothesis that “neurons that fire together wire together” (
16). Subsequent work has identified the molecular processes that underlie this neuroplasticity (
17,
18).
The hippocampus receives sensory inputs from higher-order association regions of the parietal, temporal, and frontal cortex. These multisensory inputs arrive at the entorhinal cortex, which is considered the highest level of sensory association cortex in the brain. The major route for input to the hippocampus is via the perforant path, which carries polymodal sensory information from superficial layers of entorhinal cortex and is so named because axons coming from the entorhinal cortex actually perforate the hippocampal fissure on their way to terminating in the dentate gyrus. Axon terminals from the entorhinal cortex synapse primarily on dendritic spines of granule cells in the molecular layer of the dentate gyrus, although a smaller number of entorhinal projections directly target neurons of the CA1 and CA3. Axons from dentate granule cells form a projection called the mossy fibers and synapse on the pyramidal cells of CA3. A defining feature of CA3 pyramidal cells is that they send and receive recurrent collaterals to and from many other CA3 cells. Neurons in CA3 then project to CA1 pyramidal neurons via the Schaffer collaterals. As the last step in the trisynaptic loop, CA1 axons project back to the deep layers of the entorhinal cortex, both directly and via the subiculum (
Figure 1B). While the granule cells of the dentate gyrus and the pyramidal cells of the CA3 and CA1 are excitatory glutamatergic neurons, the hippocampus also contains many different types of GABAergic interneurons (at least 20 different kinds in CA1 alone), providing a glimpse of the complexity of the circuits of the hippocampal system (
19,
20). The organization of this circuitry—funneling sensory information into the hippocampus and hippocampal projections back to the same sensory regions, giving rise to the initial input—is thought to allow for the consolidation and long-term storage of declarative memory (
5).
The selective role of the hippocampus in memory was not appreciated until 1953, when Scoville performed an experimental brain surgery on a 27-year-old patient with epilepsy, removing most of his hippocampal formation from both hemispheres. “Patient HM,” as the famous patient came to be known, became severely impaired after the procedure, suffering from anterograde as well as retrograde amnesia (
21). However, HM remained capable of recalling facts and remote memories from the years before the surgery, showed no significant change in personality or intelligence, and remained able to acquire new motor skills despite having no memory of ever having learned the skills (
22). A relatively recent discovery was the existence of a functional gradient along the long axis of the hippocampus (
23,
24). While the precise organization of function along this axis of the hippocampus is still debated, gene expression data, intrinsic and extrinsic connectivity, and behavioral dissociations make it clear that the anterior hippocampus (ventral hippocampus in rodents) is involved in avoidance behavior, unconditioned fear responses, negative valence, and anxiety (
24–
29). Gray and McNaughton (
30) were among the first to propose that the hippocampal system was central to anxiety processing.
In a very general way, the architecture of the hippocampal circuitry functions to bind together higher-order sensory information in time and space to form conjunctive, or declarative memories. Neuroplasticity within and across these different hippocampal regions is thought to subserve these memory functions. The canonical trisynaptic circuit exists along the transverse axis of the hippocampus, orthogonal to the longitudinal axis, and can be observed all along its anterior posterior extent. The functional organization of the hippocampus along its longitudinal axis is the direct consequence of the different processing streams arriving at different points along the longitudinal axis. Specifically, amygdalar inputs to the hippocampus target primarily the anterior one-third of the hippocampus, and the entorhinal regions that provide input to the anterior hippocampus also receive direct input from the amygdala (
31). At the other end, the posterior hippocampus (dorsal hippocampus in rodents) is the primary target for sensory regions carrying information about the external environment (visual, auditory, vestibular, etc.).
Another unique feature of the hippocampus is that it is one of only a few locations in the adult brain where new neurons continue to be born (
32). Consistent with this, work by Hen and colleagues has demonstrated in rodents that the antidepressant-related behavioral effects of drugs like selective serotonin reuptake inhibitors are blocked by suppressing hippocampal neurogenesis (
33). Neurogenesis has also been shown to increase with pharmacological antidepressant treatments (
34) and with exercise (
35). The finding that antidepressant efficacy is reduced by interfering with neurogenesis suggests that neuroplasticity within the hippocampus is critical for the maintenance of mood. Whether this phenomenon is central to the pathology of mood disorders or a secondary effect of antidepressant treatment, and whether these rodent findings translate to humans, remains to be determined. It should be noted that whether neurogenesis in the dentate gyrus in humans continues after childhood into adulthood has recently been called into question (
36,
37). Below we briefly review several different lines of evidence that implicate hippocampal dysfunction in the etiology of various forms of psychopathology.
It is important to note that chronic stress leads to reductions in neurogenesis, hippocampal atrophy, and lower levels of neurotrophic factors (e.g., brain-derived neurotrophic factor) in the hippocampus, and data from Sapolsky, McEwen, and many others have demonstrated the fundamental role of the hippocampus in the negative feedback loop of the hypothalamic-pituitary-adrenal axis (
38). The effects of stress and glucocorticoids on hippocampal size and function has gained much interest in recent years, especially in relation to posttraumatic stress disorder (PTSD) and major depression. An identical-twin study of hippocampal size (
39), in which one twin had been exposed to combat and the other had not, demonstrated a strong positive correlation in hippocampal size between twins. Importantly, the intensity of PTSD symptoms in combat-exposed twins was predicted by the volume of the non-combat-exposed twin’s hippocampus. The data from the study suggest that hippocampal size is familial and is a risk factor for the later development of PTSD. In vivo and postmortem studies of patients with depression often report hippocampal atrophy (
40,
41).
Intensive research over the past several decades has implicated hippocampal dysfunction in the pathophysiology of schizophrenia (
42–
45). Based on MRI morphometry and postmortem studies, hippocampal atrophy is commonly associated with the disease (
46,
47). Functional imaging studies also report abnormalities in hippocampal activation patterns in patients with schizophrenia (
48), especially in relation to tasks that probe declarative or working memory. Advances in molecular biology, human postmortem tissue collection, and animal models have focused the search for aberrant disease-related mechanisms in the hippocampus on the synaptic processes within the trisynaptic circuit (
49). This large body of research, recently reviewed by Tamminga and colleagues (
48), has led to a subfield-specific hypoglutamatergic hypothesis in hippocampal pathophysiology underlying schizophrenia. A multitude of synaptic and neuroplasticity processes have been implicated, including NMDA-dependent neurotransmission, synaptic proteins, and neurogenesis. Still other evidence points to alterations in GABAergic mechanisms in the hippocampus in schizophrenia (
43). Relevant to the present review, although not as substantial as the body of molecular work pointing to hippocampal dysfunction in schizophrenia, the evidence implicating other MTL structures, including the amygdala, is mounting (
48).
Amygdalar Anatomy, Function, and Dysfunction
The amygdala, discovered in the 19th century, is an evolutionarily conserved structure located deep within the MTL and is a key limbic region involved in assessing salience and in the processing of emotion (
50,
51). Research has demonstrated that the amygdala comprises a group of heterogeneous but highly interconnected nuclei. Although there continues to be some disagreement about the number of amygdala nuclei and their classification (
52,
53), as more data have become available, it is generally accepted that the amygdala is composed of at least seven nuclei in addition to associated cortex-like areas and transition zones (
Figure 1C). Within the amygdala, the basal, lateral, and basomedial nuclei are termed the basolateral complex (BLA) (
54,
55). These nuclei are thought to be cortical-like structures, as they are composed of both glutamatergic neurons and GABAergic interneurons and have developmental origins that are similar to those of the cortex (
56–
58). The almond-shaped BLA was first identified and described by Burdach (
51). The central (Ce) and medial amygdala nuclei, which in humans and nonhuman primates are located dorsal to the BLA, are considered striatal-like, as they are primarily composed of medium spiny GABAergic neurons and developmentally arise from the same areas as the striatum (
53,
59–
62). The cortical amygdala nucleus and the nucleus of the lateral olfactory tract, which can be found along the medial side of the MTL in primates, are laminated and are highly similar in structure and connectivity to the olfactory cortex (
53). In addition to these discrete nuclei, clusters of small GABAergic neurons exist (
63,
64). These intercalated cell masses are located between the BLA and the Ce, in primates appear as a “net” surrounding the BLA, and are implicated in gating information transfer from the PFC and BLA to the Ce (
64–
66).
Research in rodents suggests that the amygdala is composed of interacting anatomical and functional networks. In general, information flows into the BLA, which, via the BLA’s projections, is then conveyed to the Ce, where the information is distributed to downstream targets that facilitate behavioral, emotional, and physiological responses (
12,
29,
67–
69). The amygdala is associated with a variety of functions, including the encoding of both positive and negative valence (
70,
71). More specifically, the BLA encodes cues associated with reward as well as danger, and the Ce mediates the behavioral and physiological responses to threat or pain (
70,
72–
75). Our understanding of the causal role of different amygdala nuclei has been largely based on mechanistic animal studies examining adaptive threat-related behaviors. Broadly, these behaviors are characterized as unconditioned responses to threatening stimuli and conditioned responses to cues that are predictive of threat (
76–
78). In rodent studies, a common behavioral paradigm used to probe the role of the amygdala in conditioned threat behaviors was pioneered by LeDoux (
79). This paradigm utilizes Pavlov’s fundamental conditioning methods, in which an unconditioned neutral cue (e.g., light or tone) is paired with a negative or harmful outcome such as a mild shock. These studies renewed interest in understanding the function of different amygdala nuclei in learning, memory, and the expression of fear and anxiety-like behaviors and have resulted in a wealth of knowledge about amygdala microcircuits and behavior (
71,
75,
80,
81). It is important to point out that the animal studies measure observable threat-related defensive or adaptive responses, which are similar to those expressed in humans (
76). However, it is only humans who can report what their experience of fear and anxiety feels like. Thus, while the animal research addresses mechanisms associated with threat-related defensive responses, it cannot address emotional states as reported by humans (
3,
82).
The BLA receives information about the environment from the rest of the brain, such as the PFC, auditory, visual, and somatosensory cortices, the thalamus, and the hippocampus, and it is a key amygdalar structure in cued threat conditioning (
77,
83–
87). BLA nuclei send glutamatergic projections to the Ce, as well as other brain regions, and transmit the learned associations between cue, context, and threat to the Ce (
Figure 1D) (
70,
73,
88–
90). The Ce can be further divided into subnuclei. Of these, the medial Ce (CeM) serves as the major amygdala output nucleus, projecting to downstream regions such as the brainstem and the hypothalamus (
67). The lateral Ce (CeL), which largely projects to the CeM and bed nucleus of the stria terminalis (BST), is thought to act as an integrator of BLA information (
29,
90,
91). The CeL modulates the gain and gates the output of the CeM, ultimately mediating the expression of behavioral and physiological responses to threats (
91,
92).
A notable case study that exemplifies the amygdala’s role in both threat processing and conscious fear involves patient SM, who lost her left and right amygdalae to Urbach-Wiethe disease, a rare genetic condition that causes selective calcification of the amygdala. In a series of studies, SM was found to abnormally experience, express, or perceive fear (
93–
95). An interesting feature of SM is that she displays a relative reduction in the amount of time she fixates on eyes when viewing facial expressions. Since the eye region provides cues that are highly salient to fearful expressions, this could account for her difficulty in recognizing when others are fearful (
93). This observation was one of the first clues into the role of the human amygdala in salience assessment and selective attention (
96). Since then, several studies have demonstrated that the amygdala responds to a variety of stimuli (
97–
99), including the whites of the eyes (
100,
101) and visual and auditory cues associated with rewards or threats (
70,
102–
105). In addition, the amygdala is part of the salience network, as defined from fMRI studies, which is consistent with the role of the amygdala in detecting and encoding salience (
106,
107).
Although not included in the definition of the limbic system, growing evidence implicates the BST as being important in mediating threat-related behavior and fear-related psychopathology (
108,
109). The BST is found dorsal and anterior to the rest of the amygdala, underneath the caudate and behind the nucleus accumbens, where the internal capsule and anterior commissure meet (see
Figure 1C). Early work by Johnston in 1923 noted that the cell type composition, developmental origins, and connectivity of the BST, as well as the cell columns of the sublenticular region of the basal forebrain, share similarities to those of the Ce (
110). Based on these observations, the anatomist Heimer argued for extending the definition of the amygdala to include the BST and the cell columns of the sublenticular region that appear to bridge the dorsal amygdala and the BST (
111,
112). In their seminal paper, Alheid and Heimer (
113) coined the term “extended amygdala,” which recognizes the structural and functional similarities of the Ce and medial amygdala regions with the more anteriorly located BST. Although this term also incited intense debate (
111,
114), recent studies in humans, monkeys, and rodents all point to the involvement of the BST in threat detection and reward processing (
108,
109,
115) and suggest that the extended amygdala should also be considered part of the limbic system.
With regard to amygdala circuitry, information primarily flows out of the Ce and BLA to the BST (
Figure 1D) (
29,
116,
117). The BST projects to brain regions similar to those the Ce projects to, including the brainstem and hypothalamus (
108,
118). Work from Davis’s group suggested dissociable functions of the BST and Ce in mediating threat responses to different types of cues. These studies led to an influential hypothesis positing that the BST mediates sustained “anxiety-like” responses to diffuse threats, whereas the Ce was considered to underlie immediate “fear-like” responses to explicit threats (
119,
120). Recently this notion has been challenged, as evidence from human and nonhuman primate studies demonstrates that the BST and Ce interactively function to coordinate anxiety and fear-like responses (
121,
122).
Maladaptive responses to threat and amygdala activation are both hallmarks of anxiety disorders and are also associated with PTSD and specific phobias (
123). Many of these disorders arise early in life and can manifest as childhood behavioral inhibition. The concept of behavioral inhibition was pioneered by Kagan, who observed that in response to strangers and novelty, some children exhibited extreme shyness, avoidance behaviors, and increased pituitary-adrenal activity (
124–
126). This phenotype is considered a risk factor for the development of anxiety and depressive disorders later in life (
127,
128). fMRI studies examining brain activation in response to novel or fearful faces demonstrated that adults and adolescents with a history of childhood behavioral inhibition had increased amygdala activation (
126,
129). Later work from the laboratory of Blackford demonstrated that highly inhibited young adults with previously reported behavioral inhibition had increased amygdala volume (
130), faster (
131) and sustained amygdala responses to novel faces (
132), and decreased amygdala habituation (
133,
134).
Our own work has focused on understanding the neural circuitry underlying extreme anxiety by developing, validating, and testing circuit-based molecular hypotheses in a model of behavioral inhibition in young rhesus monkeys (
122,
135–
141). To evaluate behavioral inhibition in rhesus monkeys, we use the no-eye-contact condition of the human intruder paradigm, in which freezing and vocalizations are assessed in response to a potential threat. This potential threat is elicited by a human intruder presenting their profile and specifically avoiding eye contact with the subject monkey. In response to the no-eye-contact condition, monkeys typically exhibit increased orientation to the human intruder, increases in freezing behavior, and decreases in coo vocalizations, along with increases in cortisol levels. We conceptualize these behavioral and physiological threat responses as a composite measure of anxious temperament and a model of the childhood risk of developing anxiety disorders (
127,
142). We have investigated the neural circuitry of anxious temperament in a large pedigree of young animals and found that greater metabolism in the extended amygdala, both in the Ce and the BST, is associated with increased anxious temperament. Our work highlights the fact that the extended amygdala is part of a larger anxious temperament–related circuit that includes the posterior OFC, anterior hippocampus, and brainstem nuclei, such as the periaqueductal gray, that together work to promote survival in response to threat (
143). Alterations in this brain circuit have been implicated in altered emotion processing across various psychiatric disorders, including the predisposition for anxiety, anxiety disorders, depression, schizophrenia, and autism spectrum disorder (
144).
PFC Anatomy, Function, and Dysfunction
The PFC, located at the rostral aspect of the frontal lobe, is considered the seat of higher cognition, subjective emotional experience, and personality. The scientific study of the structure and function of the human PFC arguably began with the accident that made railroad worker Phineas Gage famous after he survived an explosion in which an iron tamping rod penetrated his frontal lobes, resulting in personality change (
145,
146). Since the time of Von Economo and Brodmann, neuroanatomists have been mapping the regional differences in cytoarchitecture and connectivity that define different areas of the PFC. But exactly which cortical areas comprise distinct PFC regions has been another area of debate (
147–
150). The PFC can be roughly divided into four major subregions: the orbitofrontal cortex (OFC) on the ventral surface (Brodmann’s areas [BA] 10–14), the medial prefrontal cortex (mPFC; BA 10, 11, 14), which some anatomists include together with the anterior cingulate cortex (ACC; BA 32, 24, 25), and the ventrolateral (vlPFC; BA 10, 12, 47) and dorsolateral (dlPFC; BA 8, 9, 45, 46) prefrontal cortices (see
Figure 1E). These PFC regions are key structures constituting cortical and subcortical networks underlying a host of functions, such as mediating value and reward-based decision making (
151,
152), emotion regulation (
153), cognition (
154), theory of mind (
155), and abstraction and working memory (
156). The PFC is the most evolutionarily recent brain region to develop, and it is relatively larger in humans compared with other mammals (
157,
158). Compared with other parts of the brain, the PFC has a protracted postnatal development, continuing to grow in size and complexity throughout adolescence (
159–
161). Additionally, the white matter in the frontal lobes is the last to become myelinated (
162).
Price and colleagues proposed a dual network organization in the OFC/mPFC (
148), such that in the primate brain there exists a medial network, strongly interconnected with visceromotor brain areas, and an orbital network that is interconnected with polymodal sensory regions. They also proposed a lateral network that includes the dorsal surface of the PFC (
163). The structures of the MTL primarily target the medial network, but they also target the dysgranular regions of the posterior OFC and the perigenual ACC (BA 24, 25), and these connections are implicated in the pathophysiology of mood disorders (
163). By mapping the structural connectivity between the MTL/anterior temporal lobe and the PFC, recent diffusion tensor imaging tractography results from monkeys and humans lend anatomical support to this hypothesized dichotomous network organization of the OFC/mPFC (
164,
165). The ventral amygdalofugal pathway connects the MTL with mPFC regions, whereas the uncinate fasciculus appears to primarily connect the anterior temporal cortex with the orbital surface and vlPFC regions. These anatomical dissociations are critical because different forms of psychopathology could depend on the connections carried by the amygdalofugal pathway, for example, while others may depend on alterations carried by uncinate fasciculus fibers, or still other pathways carrying long-range axons from other brain areas.
As part of the medial network, alterations in vmPFC function have been primarily associated with anxiety and affective disorders, whereas the dlPFC has been a primary focus of research on schizophrenia. Abnormalities in the cytoarchitecture of the dlPFC have been reported in schizophrenia, and recently specific alterations in parvalbumin-expressing GABAergic interneurons in the dlPFC have been implicated in the cognitive deficits associated with the illness. These data lend support to the hypothesis that GABAergic neurotransmission in the dlPFC is related to the pathophysiology of schizophrenia (
166). While these PFC regions are important, it is the connections between the MTL and PFC that coordinate cortical-limbic interactions that are particularly relevant to the pathophysiological processes underlying psychopathology (
167,
168). Below we highlight findings that implicate the connectivity between the MTL and PFC in the pathophysiology of psychiatric illnesses.
PFC-MTL Circuitry in Adaptive Behavior and Psychopathology
Based on animal work, it is clear that the PFC, hippocampus, and amygdala mediate various aspects of adaptive functioning necessary for survival. However, the PFC and MTL form complex, interacting circuits, which process and integrate information to facilitate cognitive-emotion interactions and the selection of appropriate behavioral responses. Here we discuss human neuroimaging work in light of cross-species tract tracing and behavioral studies that together provide compelling exemplars of PFC-MTL interactions that appear to be altered in psychopathology (
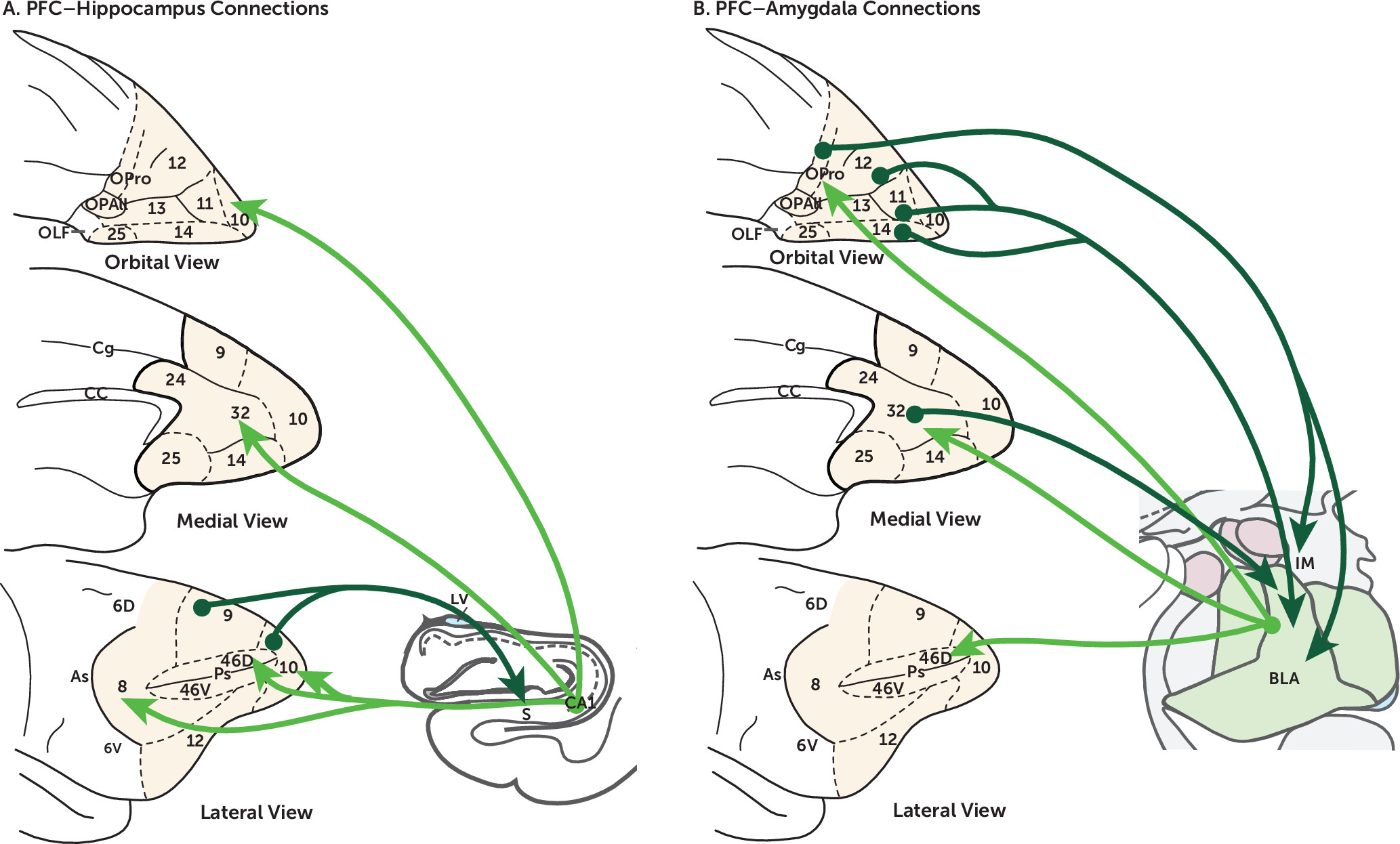
Figure 2).
The OFC and mPFC send robust projections to the BLA, and the BLA in turn sends reciprocal projections back to these areas (
169,
170). Studies demonstrate that these circuits mediate value-based and reward-based decision making (
171,
172), guide the selection of behaviors in the presence of conflicting cues (
173), and have been implicated in the cognitive control of emotion. The ability to make appropriate decisions based on potential outcomes is an adaptive capacity often altered in psychopathology. This is exemplified by indecisiveness and inaction, which are common in individuals suffering from major depression and anxiety disorders (
174). Impaired regulation of innate and/or learned fear responses is another feature of anxiety-related disorders. Consistent with this, human neuroimaging studies demonstrate that the extinction of fear memory is dependent on the ventral mPFC and involves the amygdala and hippocampus (
175–
177). Extinction learning is impaired in patients with PTSD, and fMRI activation in the hippocampus and mPFC during extinction recall is decreased in patients with PTSD (
178). The PFC communicates with the MTL in part through the uncinate fasciculus, and recent evidence suggests that decreased microstructural integrity of the uncinate fasciculus is associated with anxiety disorders in children and adults (
179–
181). These studies are among those suggesting that limbic connections with the mPFC are central to the impaired learning and cognitive distortions associated with depression, PTSD, and other stress-related disorders (
40,
127).
Another example of PFC-MTL connectivity likely involved in psychopathology is the direct projections from the anterior hippocampus, originating from CA1, subiculum, and prosubiculum, to the PFC (
182). In nonhuman primates, the most robust hippocampal projections to the PFC terminate in the medial and orbital networks of the OFC/mPFC (
183,
184). Also, neurons in the dlPFC have been identified that project back to the hippocampus (
185). While the projections from the hippocampus to the PFC may be more important in spatial working memory and defensive behaviors (
186,
187), it is the projection from the dlPFC to the hippocampus, via the subiculum and parahippocampal cortex, that may be important in the cognitive control of emotional memory retrieval (
188). These pathways may be particularly relevant to illnesses such as schizophrenia that involve impairments in working memory and in which hippocampal and PFC alterations occur.
Together, these studies point to the importance of PFC-MTL connectivity in adaptive functioning and highlight the importance of understanding alterations in this circuitry in relation to psychopathology. At a clinical level, understanding the structural and functional connections within and between these circuits provides the foundation for novel therapeutic strategies. Current treatment strategies aimed at modifying neural circuit function, such as transcranial magnetic stimulation (TMS), a noninvasive method to modulate neuronal function, and deep brain stimulation (DBS), presumably work in part via their effects on modulating PFC-MTL circuits (
3,
189). Several studies have demonstrated that TMS directed at the dlPFC can lead to improvements in PTSD and depression symptoms (
190). Recent work from Etkin’s lab found that PTSD patients with exposure treatment–related symptom reductions demonstrated increased dlPFC activation and decreased amygdala activation (
189). The researchers also found that a TMS pulse applied to the dlPFC was more effective in reducing amygdala fMRI activation in these patients relative to waiting-list control subjects at baseline (
189). Finally, Mayberg has pioneered the use of DBS of the subcallosal ACC (BA 25) to treat refractory depression, and although a recent clinical trial failed (
191), open-label use of DBS has been successful in some patients with refractory illness (
192). BA 25 is a critical hub connecting many parts of the PFC, MTL, and autonomic brainstem centers (
193), and the structural connectivity of this region has become a focus in the search for the mechanisms underlying the therapeutic efficacy of subcallosal DBS (
194,
195).
Conclusions
Human neuroimaging and postmortem studies demonstrate that PFC and limbic system functions are altered in psychopathology. However, findings from individual studies vary. Meta-analyses of neuroimaging studies point to shared circuit alterations across disorders, as well as some specificity (
123,
196). For example, threat-related amygdala activation is similarly elevated in individuals with social anxiety disorder, PTSD, or specific phobias (
123). Additionally, gray matter reductions and alterations in the frontoparietal control networks are also common across many disorders, including schizophrenia and bipolar disorder (
197). These findings are consistent with the high levels of comorbidity and the overlap in symptoms that occurs across different psychiatric diagnoses (
198). Another issue pointing to the overlap across disorders involves treatment. Similar medications are used to treat shared symptomatology across diagnoses. For example, the same medication class (i.e., selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors) are effective for different illnesses, such as anxiety disorders and depression. Taken together, the high levels of comorbidity, shared symptoms, and similar pharmacological responsivity highlight major challenges regarding how we understand mechanisms underlying pathophysiology in relation to diagnoses. As an alternative to the traditional DSM categorical approach, in 2012 the National Institute of Mental Health proposed the Research Domain Criteria (RDoC) project with the aim of linking the dimensionality of symptoms, regardless of diagnosis, to associated brain circuits involved in adaptive functioning (
199). While there has been considerable debate as to the pros and cons of these approaches (
200,
201), given the current state of our knowledge, combining categorical and dimensional strategies will yield an integrated approach with the greatest likelihood of truly reflecting the nuances and complexities of psychiatric illnesses.
Understanding the mechanistic role of the limbic system and its interactions with the PFC in different aspects of psychopathology is a major challenge for the field of psychiatry. To try to bridge this gap, researchers using animal studies have focused on understanding the anatomy and function of regions of the limbic system in the context of adaptive behaviors that may be altered in psychopathology (e.g., appropriate responses to threat, goal-directed behavior, and working memory function) and the ways in which MTL-PFC projections regulate these behaviors (
28). It is our hope that a better understanding of these circuits, combined with recent advances in neuroscience methods, will provide the basis for clinical and preclinical research focused on new ways of classifying patients on the basis of brain circuit dysfunction, identifying the relationships between circuit dysfunction and symptoms, and exploring novel treatment approaches.