Multiple lines of evidence implicate the thalamus in psychotic disorders, including smaller thalamus volume, decreased activation during task performance, abnormal functional and anatomical connectivity with the cortex, reduced expression of biochemical markers of neuronal integrity, abnormal sleep spindles, and lower cell numbers in some thalamic nuclei (
1–
13). These findings contributed to the development of several models of psychosis, many of which propose a neurodevelopmental basis for thalamus pathology and emphasize thalamic abnormalities in the mechanisms of cognitive impairment (
14–
18).
Several models further propose selective dysfunction of specific thalamic nuclei, particularly thalamic association nuclei, including the mediodorsal and pulvinar nuclei (
14–
18). There is abundant evidence that connectivity of some association nuclei (e.g., the mediodorsal nucleus) are abnormal (
3,
4); however, evidence of selective anatomical abnormalities is sparse and inconsistent. For instance, while postmortem studies consistently find smaller volume and lower cell numbers in the pulvinar (
19–
21), mediodorsal nucleus findings are mixed (
22,
23), and small sample sizes raise broader concerns about replicability and generalization from postmortem studies (
10,
23). Similarly, the handful of neuroimaging studies examining specific thalamic nuclei report both smaller and normal mediodorsal and pulvinar volumes (
24–
27). Inconsistent neuroimaging results are likely due to a combination of factors, including modest sample sizes and use of idiosyncratic methods for quantifying thalamic nuclei that have not been widely adopted by the neuroimaging community.
The recent development of a novel method for segmenting the thalamus has created a new opportunity to investigate the thalamus in psychosis. Specifically, adapting an approach developed to segment hippocampal subfields (
28), Iglesias and colleagues (
29) built a probabilistic atlas from ex vivo MRI and histological data that can be applied to standard T
1-weighted in vivo MRI to segment thalamic nuclei using Bayesian inference. Critically, their method is able to recover the three-dimensional structure of histological data from ex vivo MRI; yields volumes that are in good agreement with other histological atlases of the thalamus; has excellent test-retest reliability for most thalamic nuclei (interclass correlation coefficients ranging from 0.86 to 0.99); is robust against changes in MRI contrast; and demonstrates good correspondence between in vivo MRI and established neuropathology in neurological disorders (i.e., Alzheimer’s disease). Moreover, their method is included in FreeSurfer, one of the most widely used software packages for quantifying brain anatomy, ensuring dissemination to the broader neuroimaging community (
29).
We applied the method described above to a large cohort of individuals with psychotic disorders (N>450) and the Philadelphia Neurodevelopmental Cohort (PNC) (N=1,601), which includes youths with psychosis spectrum symptoms, in order to clarify the anatomical specificity, neurodevelopmental basis, and cognitive correlates of thalamic pathology in psychosis. Our investigation had three aims. First, we sought to characterize thalamic nuclei volumes in psychosis; we hypothesized that thalamic mediodorsal and pulvinar nuclei would be smaller in psychosis. Second, we sought to determine whether thalamic abnormalities extend to youths with psychosis spectrum symptoms and to establish whether thalamic volume abnormalities are specific to psychosis or are related to psychopathology more broadly. Consistent with a neurodevelopmental basis for thalamic pathology in psychosis, we hypothesized that youths with psychosis spectrum symptoms, but not youths with other psychopathologies, would demonstrate a pattern of volume abnormality similar to that of individuals with psychotic disorders. And third, we sought to establish the cognitive correlates of thalamic nuclei volumes in individuals with a psychotic disorder and youths with psychosis spectrum symptoms. In keeping with models proposing thalamic dysfunction in the mechanisms of cognitive impairment in psychosis, as well as abundant evidence demonstrating the importance of the mediodorsal and pulvinar nuclei in higher cognitive abilities (
30–
32), we hypothesized that the volume of the mediodorsal and pulvinar nuclei would correlate with cognitive function across typically developing individuals, individuals with psychosis, and youths with psychosis spectrum symptoms.
Discussion
We examined thalamic nuclei volumes in a large cohort of individuals with psychotic disorders and a community-ascertained neurodevelopmental cohort, the PNC. We used a recently developed, validated method included in the FreeSurfer software package for segmenting thalamic nuclei on conventional T1-weighted MRI and complemented this approach with a VBM analysis.
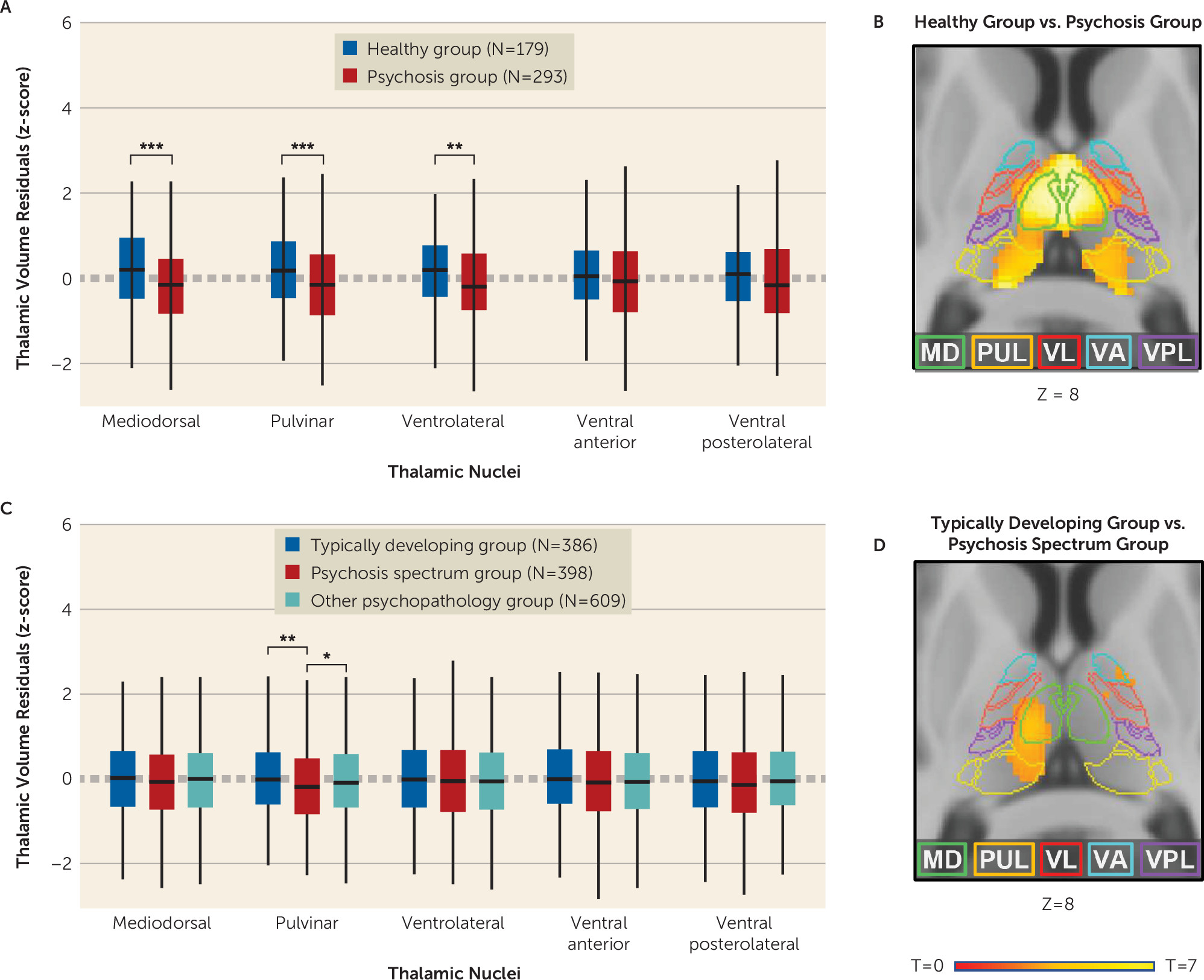
The study findings contribute to our understanding of thalamic pathology in psychosis in several ways. First, they clarify the anatomical specificity of thalamic structural abnormalities in psychosis. Using complementary segmentation and voxel-based approaches, we found that lower thalamic volume was most pronounced in the mediodorsal and pulvinar nuclei. As touched upon earlier, findings from postmortem (
10) and neuroimaging studies (
24–
27) are mixed, likely because of a combination of several factors, including small sample sizes and, in the case of neuroimaging studies, variable methods used to measure specific thalamic nuclei. Our results support thalamic models of psychotic disorders that emphasize dysfunction among association nuclei.
Second, our finding that youths with psychosis spectrum symptoms also demonstrate smaller thalamic volumes is consistent with a neurodevelopmental basis for thalamic abnormalities in psychosis (
14,
15). Disruption of the thalamus during development may affect cortical development in regions involved in the pathogenesis of psychosis (e.g., the prefrontal cortex). For instance, animal studies show that disrupted thalamus development is associated with lower cell density and volume in neuroanatomically connected cortical regions (
43,
44). In our neurodevelopmental cohort, smaller pulvinar volume was specific to the psychosis spectrum group. Smaller pulvinar volumes were constant across age in both samples, suggesting that structural abnormalities in the pulvinar are present prior to the onset of psychosis. VBM analysis indicated that smaller volume in the psychosis spectrum group also encompassed a portion of the mediodorsal nucleus. Indeed, qualitative comparison of VBM results indicates that there is significant overlap in thalamic volume loss in the psychosis sample and in the psychosis spectrum youth sample, although, not surprisingly, volume loss is less extensive in the psychosis spectrum group.
While previous studies have identified smaller thalamic volume in youths exhibiting symptoms of psychosis, including individuals at clinical high risk (
45,
46), the present results extend these findings in critical ways. First, we included a large cohort of individuals with psychotic disorders to compare concordance across the psychosis spectrum. Second, we examined volumes of specific thalamic nuclei, in contrast to most previous studies, which examined whole thalamus volumes only. Third, our sample size is considerably larger than those of many previous studies, including a recent study that used an earlier release of the PNC data that did not include the complete neuroimaging sample (N=997, compared with N=1,601 in the present study) and separated youths with psychosis spectrum symptoms only from those with psychosis and bipolar spectrum symptoms (
47). Fourth, in contrast to all previous studies (
47,
48), we established that smaller thalamic volume is specific to youths with psychosis spectrum symptoms by including a large sample of youths with other psychopathology. Finally, we examined associations between thalamus volume and cognitive function.
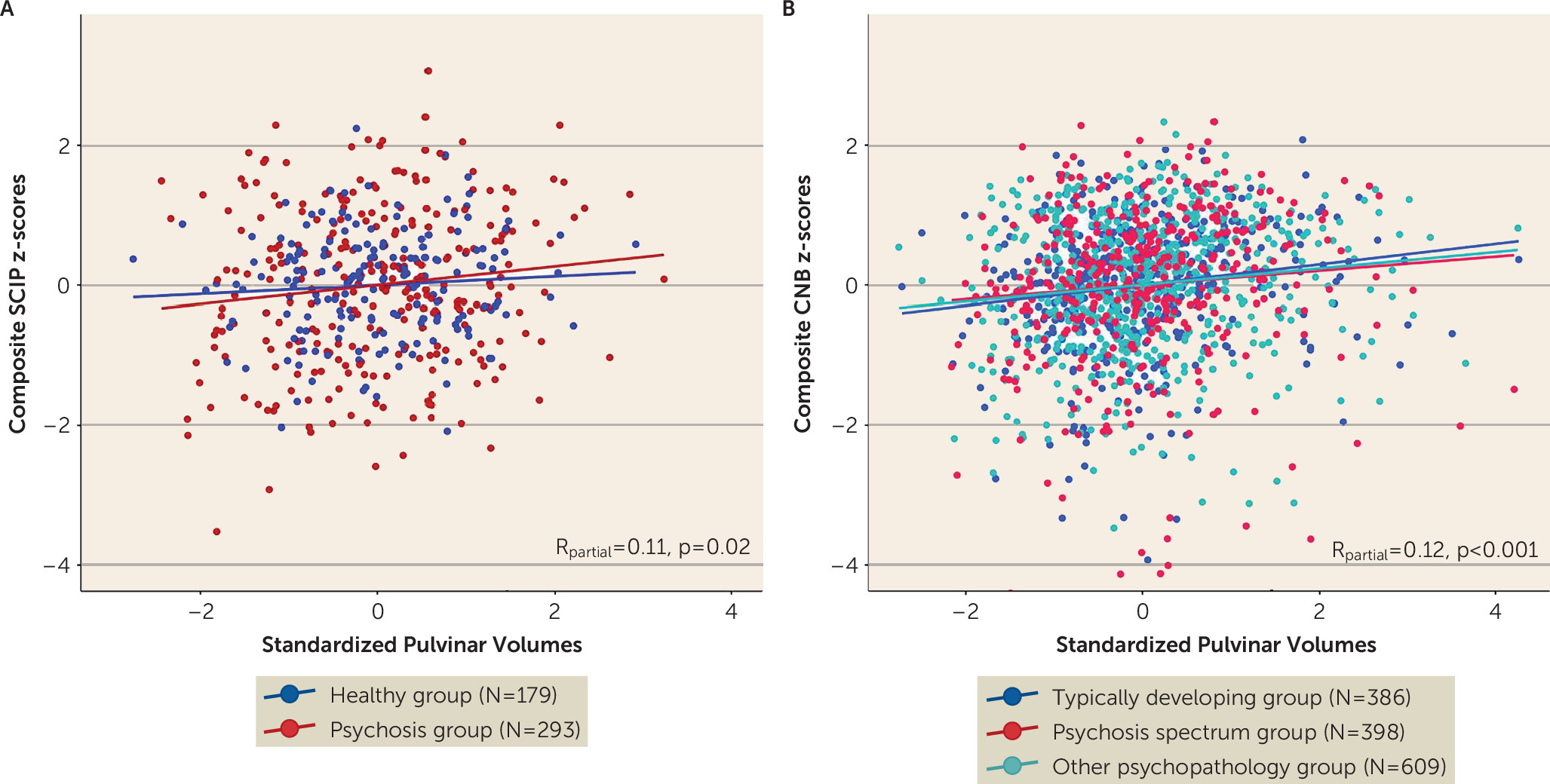
One major implication of smaller thalamic nuclei volumes in both psychosis and in youths with psychosis spectrum symptoms is that this pathological abnormality contributes to the cognitive impairment considered to be a core feature of psychotic disorders (
49). This is supported in our data, as thalamic nuclei volumes, and of the pulvinar specifically, are associated with general cognitive function. Moreover, the association, while modest, was strikingly similar across the psychosis and youth cohorts. Previous studies have found that whole thalamus volume and pulvinar-cortex covariance predict cognition in schizophrenia (
50,
51). The pulvinar is intimately involved in cognitive functions, particularly as it relates to flexible, goal-oriented direction of attention (
30). The pulvinar also has a role in synchronizing cortical activity during attention (
52,
53), and lesions of the pulvinar reduce attention-related signals in the cortex (
53), suggesting that it may influence cognition through thalamo-cortical interactions. Inappropriate modulation of attention through cortical networks that include the thalamus (e.g., the salience network) is hypothesized as a core deficit in schizophrenia that impairs the integrity of sensory information and context processing to control goal-directed behavior (
54). Furthermore, positron emission tomography studies in schizophrenia have demonstrated reduced dopamine D
2 receptor binding in the pulvinar and mediodorsal nucleus specifically, with lower binding being associated with more severe psychotic symptoms (
55,
56).
Our study had several strengths, including large sample sizes, complementary methods for measuring thalamic volumes, and convergent findings across both methods and cohorts. Nevertheless, there are several limitations. While our use of an automated segmentation technique allowed us to obtain a well-validated segmentation of larger thalamic nuclei, we did not include several nuclei because of their small size and poor contrast in standard 1-mm3 T1 imaging, some of which may be relevant to psychosis, such as the lateral and medial geniculate nuclei. Another limitation was our use of cross-sectional samples, which limits our ability to investigate changes in the volumes of thalamic nuclei over time in individuals with psychotic disorders and youths with psychosis spectrum symptoms.
Acknowledgments
This work was conducted in part using the resources of the Center for Computational Imaging at the Vanderbilt University Institute of Imaging Sciences and the Advanced Computing Center for Research and Education at Vanderbilt University.
The authors thank the individuals who participated in the study, as well as Kristan Armstrong, Erin Brosey, Molly Boyce, Victoria Fox, Yasmeen Iqbal, Margo Menkes, Austin Woolard, Katherine Seldin, and Margee Quinn for their assistance in recruiting and screening study participants.