It is useful to compare the heritability results from family-based and SNP-based approaches. Family-based studies, being more direct, typically yield estimates of heritability with lower standard errors, whereas the inaccuracy of estimating distant relationships from genetic data tends to produce fuzzier estimates. Family-based estimates also tend to yield higher estimates of heritability because the familial covariance reflects both rare and common genetic variation, whereas SNP-based estimates mostly arise from covariance due to common genetic variants. Looking at the results summarized above, one might conclude that this is also operating for OCD, that is, that family-based studies are producing higher heritability estimates than SNP-based studies.
However, in an influential study of data from the International Obsessive-Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) by Davis et al. (
5) (1,061 case subjects, 4,236 control subjects, 373,846 SNPs), there was no evidence for heritability from SNPs with minor allele frequency (MAF) <0.05, and over 60% of total heritability mapped to the most common variants (MAF >0.3). In addition, in a meta-analysis of data from the International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Study (OCGAS) (
16), ∼60% of heritability was accounted for by SNPs with MAF >0.4 in both the OCGAS sample alone and in the combined sample. If this observation were true, it could have profound implications for which evolutionary forces shaped this unusual mapping of risk alleles to their population frequency distribution. For example, balancing selection, where multiple alleles are maintained in the gene pool of a population at frequencies larger than expected from genetic drift alone, may play a role in OCD.
Discussion
Common genetic variation—variants shared among many individuals in a population, which are most frequently SNPs—has been found to play a role in liability for most psychiatric disorders, including OCD. Open questions remain about the impact on risk due to common variation, including how much of the heritability of OCD common variation accounts for and how it is partitioned across the frequency spectrum of alleles. These are important questions for a variety of reasons. For example, both schizophrenia and autism spectrum disorder demonstrate high heritability (
32,
33), and much of the heritability traces to common genetic variation. Yet, rare variation with a damaging impact on gene function, especially de novo variation, plays a larger role in overall autism spectrum disorder risk than in overall schizophrenia risk (
32,
34,
35); for example, Singh et al. (
35) found, when they evaluated evolutionarily constrained genes, that de novo protein-truncating variants were four times more common among individuals with autism than among individuals with schizophrenia. This difference is critical for clinical genetics, genetic counseling, and possibly treatment. It also could be relevant for disentangling evolutionary processes underlying different psychiatric disorders, consistent with stronger natural selection on autism than schizophrenia. Finally, it would have an impact on study design (if, for example, rare variants contribute little to OCD heritability).
Here, we evaluate whether a substantial portion of the heritability of OCD traces to common variation, as it does for autism and schizophrenia, and we characterize its frequency spectrum, which is directly relevant to evolutionary processes. For example, in an early study estimating the heritability of OCD from common variation, results from the IOCDF-GC sample (
5) suggested that alleles with the highest frequencies—that is, those with MAF >0.3—account for the bulk of SNP-based heritability of OCD. Similar findings were reported using meta-analysis of data from IOCDF-GC/OCGAS (
16). Such a strong pattern would suggest that OCD was under strong balancing selection.
By sampling individuals with OCD from the Swedish population, as well as a larger sample of unaffected (control) individuals, we were able to address these questions. Our analyses of over 2,000 individuals diagnosed with OCD and twice as many unaffected individuals, each genotyped across their genome via >400,000 SNPs, yielded an OCD heritability estimate of 29% (SE=4%), a robust estimate (
Table 3).
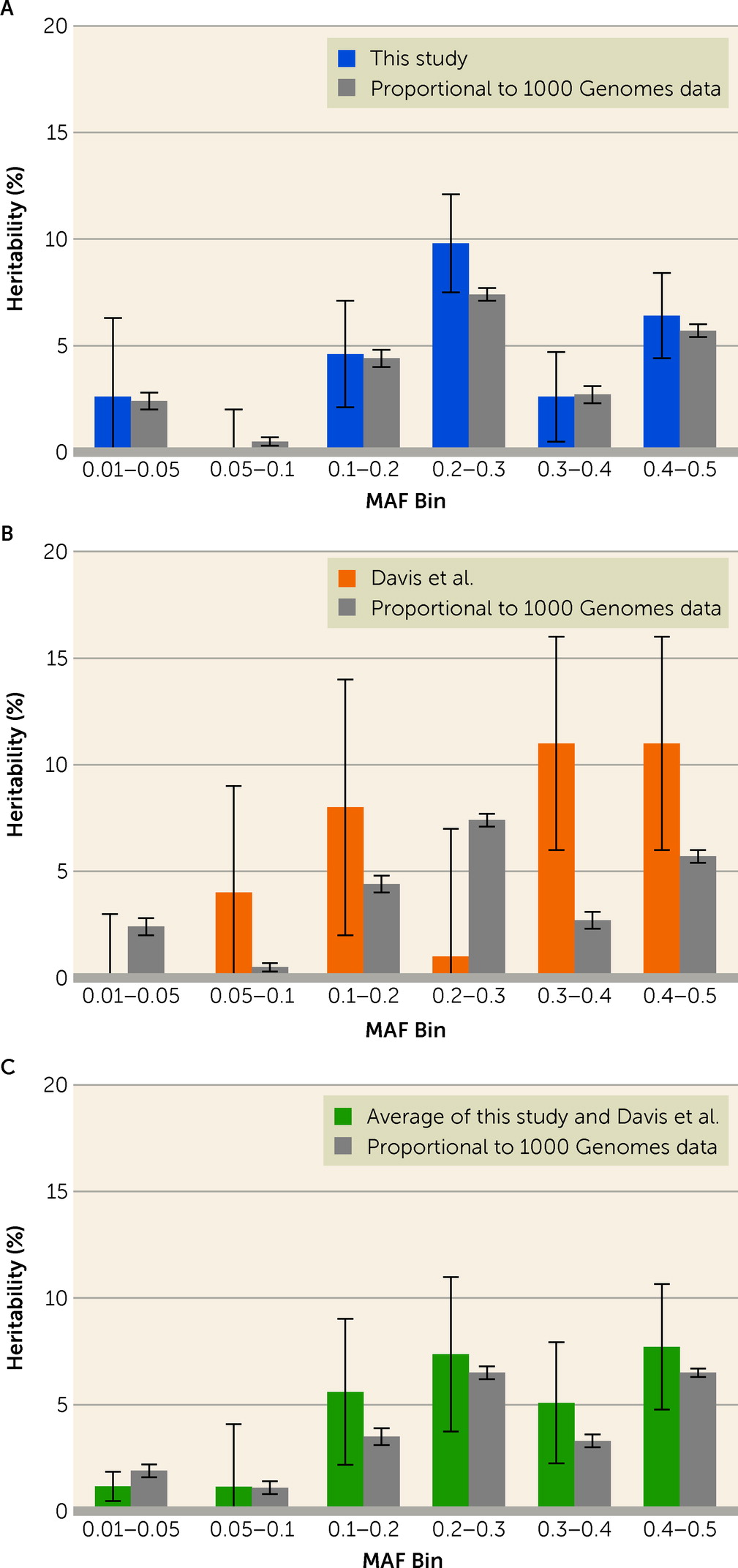
Moreover, when we assumed that SNPs contributed equally to risk for OCD, regardless of MAF, we obtained good fit between estimated OCD heritabilities from MAF bins of our sample and what was expected based on the distribution of MAF in 1000 Genomes data (
Figure 1). SNPs affecting risk appear to be distributed at random over chromosomes, because size was a good predictor of a chromosome’s contribution to total heritability (
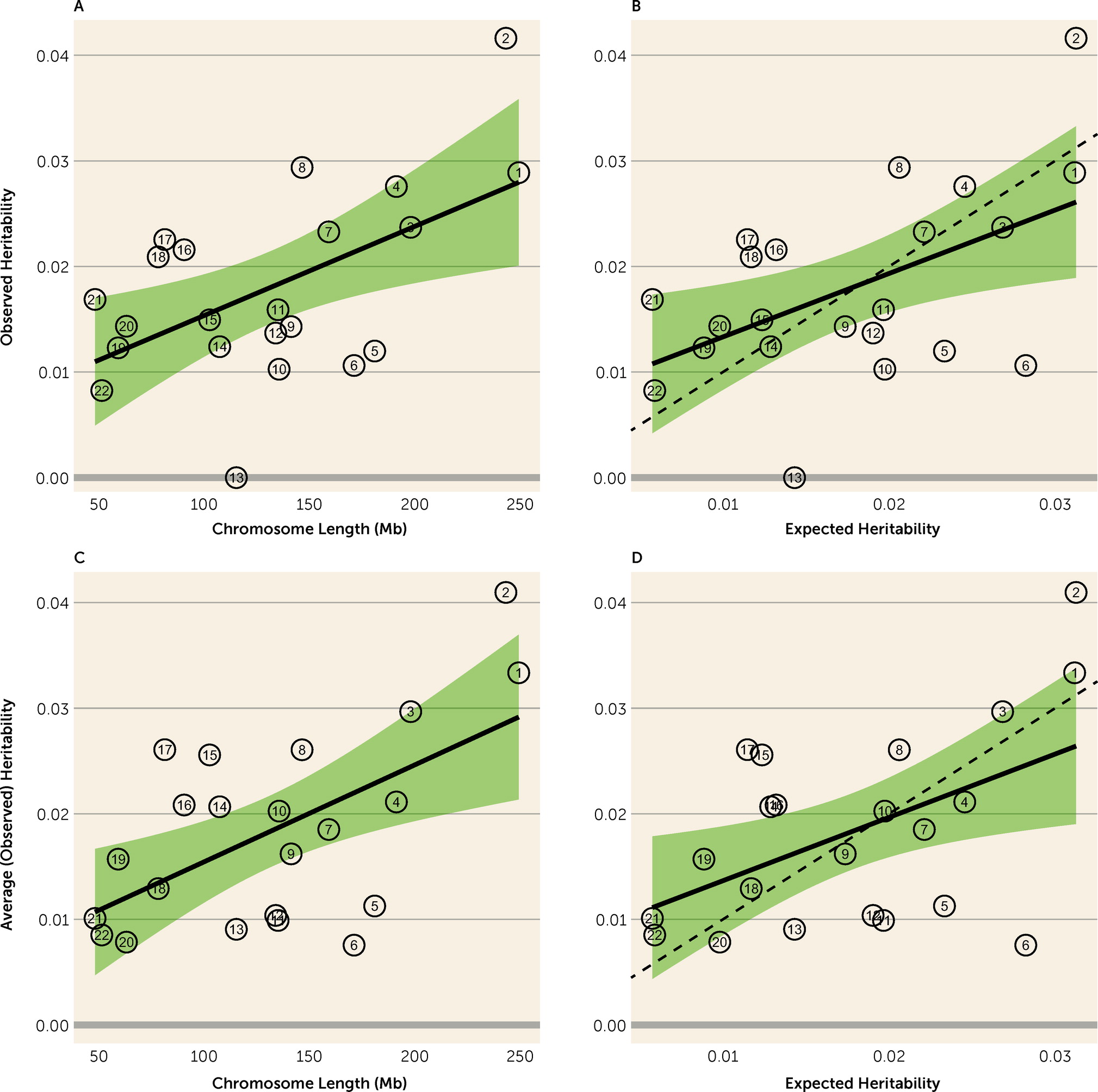
Figure 2). Chromosome 13 showed the poorest fit to this model, which may be partially explained by its having one of the lowest gene densities (6.5 genes per Mb) among human chromosomes. All of these results fit expectations of the infinitesimal quantitative genetics model.
In terms of estimated heritability from common variation, our results compare favorably with previous studies of OCD. Published estimates of SNP-based heritability, based on different samples from different populations, range from 25% to 43% (
5,
15,
16). Thus, all studies have converged on a substantial contribution of common variation to the heritability of OCD, showing notable consistency. There are some differences, however. Notably, recent studies (
5,
16) suggest that SNPs with substantial frequency (MAF >0.05) contribute disproportionately to this heritability, and the contribution tends to increase with increasing MAF.
In light of our findings, we found their results intriguing: An increasing heritability associated with MAF is appealing because the contribution to heritability of any SNP of frequency
p is
2p(1−p)a2, where the SNP’s effect
a can be assumed to be roughly equal over all SNPs under the infinitesimal model; on the other hand, it seems unlikely that low-MAF SNPs have no contribution to heritability because there are so many of them in the human genome (see Table S9 in the
online supplement). Our results argue that these low-MAF SNPs do contribute to OCD heritability, that their contribution is roughly in proportion to the frequency spectrum of alleles, and that they can be assumed to be of similar effect (i.e.,
a) across the frequency spectrum. Thus, our results show that future studies of less common and even rare alleles will also be informative on OCD etiology, with the caveat that effects of risk alleles of very low frequency can be difficult to detect by case-control methods.
Another interesting contrast is the evidence for heritability across chromosomes. Davis et al. (
5) observed essentially no heritability for OCD on chromosome 6, which encodes both the HLA and histone gene clusters, and extremely high heritability on chromosome 15. In discussing these results, the authors suggest that chromosome 15 has an outsized contribution to OCD risk and that the HLA locus is effectively excluded from OCD risk. Given the contrasting results in our study, and in our analyses combining results from both studies, we again conclude that the data are consistent with the infinitesimal model and that smaller sample sizes may account for results that diverge from expectation.
The Davis et al. study (
5) involved about 50% fewer OCD cases, and the variance in any estimate is a direct function of sample size. It is also possible that the IOCDF-GC sample had a different distribution of distantly related individuals than our relatively homogeneous sample from Sweden. The accuracy of SNP-based heritability diminishes as the fraction of very distantly related pairs, relative to all relative pairs, increases. Consistent with estimates from both studies being noisy, when we combined our results with results from the IOCDF-GC sample (
5) to obtain new estimates of average heritability per allele bin and heritability per chromosome, the average fit expectation was better than in either study alone.
Prior to the advent of dense genotyping, the heritability of a trait was typically estimated from its distribution within pedigrees. These kinds of studies continue to this day, in large part because they capture heritability due to both common and rare inherited genetic variation. It is thus interesting to compare our SNP-based heritability estimate from common variation, 29%, to that from Swedish families, 35%–50% (
1,
4). This comparison suggests that while the majority of inherited liability for OCD traces to common genetic variation, rare variation contributes to OCD liability as well, but to a lesser degree, consistent with the findings to date regarding rare variation and risk for OCD (
17–
20).
The present study had strengths and limitations. We used OCD cases from the EGOS and NORDiC cohorts, the two largest OCD studies in Sweden, to examine the role of genetic and environmental factors. The EGOS cohort utilized the Swedish National Patient Register for its sampling frame, and thus it is an epidemiological cohort, minimizing selection biases, while NORDiC recruited through specialty OCD clinics across Sweden, a sampling frame more typical of case-control studies. This difference in sampling frames could introduce heterogeneity into our study. Nonetheless, when we evaluated this possibility by estimating the heritability induced by contrasting OCD case subjects from EGOS to OCD cases from NORDiC, and doing the same for control subjects, both estimated heritabilities were not significantly different from zero. Hence, while there could be subtle heterogeneity between the cohorts, it must be small. Furthermore, for both cohorts, reliance on inclusion as a result of individuals seeking care at mental health hospitals and clinics can inadvertently exclude those with milder forms of the disorder who may seek treatment from primary care providers and those who do not present to clinical services at all. If such individuals were included, and if their genetic architecture were different from that of our current OCD case sample, it would affect the estimated heritability. By restricting case subjects to individuals in Sweden, we had a genetically homogeneous sample, which minimized the risk of confounding due to population stratification and facilitated the combining of the cohorts. Nonetheless, it does limit the generalizability of our results. However, after combining our results with those of the IOCDF-GC sample (
5), we observe results that fit expectation, thus suggesting that the results are likely to be relevant for most populations.
Our results provide new insights into the genomics architecture of OCD, affecting research design for genomic discovery and the ultimate clinical impact of such studies. While there is no doubt that rare and common genetic variation contributes to risk for OCD, the balance of their contributions has remained uncertain. The results from the IOCDF-GC sample and the meta-analysis of IOCDF-GC and OCGAS samples (
5,
16) implied an unexpectedly large role for very common variation in OCD risk and no evidence for heritability related to rarer variation (MAF <0.05). This would be quite distinct from what is known about other psychiatric disorders, and it would be consistent with some form of evolutionary selection, such as balancing selection. Our results differ substantially from those of the earlier studies; specifically, we observed that the contribution to risk from common SNP variation follows expectations. Hence, our results do not support a role for unusual evolutionary forces playing a role in OCD risk and do support a role for rare variation in risk.
Assessing the contribution of rare variants in OCD has the additional benefit of uncovering variation of major effect, which can lead to direct insight into OCD biology and potentially pave the way for family counseling. In addition, these high-effect genes represent tools to create animal models of OCD to study pathobiology and also may represent targets for developing novel therapeutics.
As data sets become larger, risk prediction will improve, as will our ability to characterize the distribution and effects of common and rare risk variation. We conjecture that the liability arising from common and rare risk variation likely combines additively to determine risk for individuals diagnosed with OCD, similar to the risk patterns for ASD (
36,
37). This knowledge can be translated into a deeper etiological understanding of OCD subtypes and their treatment and, in the future, into better predictors of OCD risk. OCD is a clinically and etiologically heterogeneous condition (
38) with a complex symptom structure (
39). Studies suggest that the burden of common risk alleles of OCD may differ by OCD symptom type. For example, although not yet replicated, in one study (
40), compulsive symptoms rather than obsessive symptoms showed higher SNP heritability and genetic correlations with OCD. However, it is still unclear to what extent rare genetic variation, and the joint effect with common variation, may 1) differ between the subtypes of OCD, 2) interact with sex, 3) influence age at onset, and 4) affect the risk of comorbid conditions. Further research in this area could accelerate discovery of biomarkers and novel treatments, eventually helping clinicians offer patients optimal prognosis and treatment.
Pharmacogenetic studies of OCD have focused on the role of common genetic variants in treatment response (
41). However, to date, no replicated significant GWAS variant has been reported for OCD—likely because of the small sample sizes—and therefore it has been challenging to contextualize the results of OCD pharmacogenetic studies. Future pharmacogenetic studies examining predictors of treatment response will likely shift focus from selected common genetic variants to genome-wide studies that also estimate how rare and common risk variants jointly affect liability (
42).
The heterogeneity of OCD should always be considered in the light of psychiatric comorbidity, an approach that is facilitated in samples such as EGOS and NORDiC that are linked to national health registries. For example, in EGOS, using an epidemiological frame, approximately 40% of individuals with OCD have more than one psychiatric comorbidity, with anxiety disorders and major depressive disorder being most common (
43). In addition, the severity of OCD was significantly higher in individuals with at least one additional psychiatric comorbidity compared with individuals with no psychiatric comorbidity: higher symptoms of obsessing and ordering, measured using the Obsessive Compulsive Inventory–Revised, were observed in individuals with OCD and at least one additional psychiatric comorbidity. In future studies, it will be important to investigate how the combinations of rare and common genetic variants differ in their relationship with the comorbid conditions.
In summary, our results demonstrate that the majority of inherited liability for OCD traces to common genetic variation, and the distribution of risk as a function of allele frequency is consistent with expectations of the infinitesimal quantitative genetics model. These results indicate that balancing selection or other, more complex evolutionary forces are not strongly at play in OCD. Furthermore, since the infinitesimal model assumes that genetic loci across the entire frequency spectrum influence the risk of OCD, future studies that examine the contribution of rare variation, both inherited and de novo, together with common variation, will allow a more comprehensive understanding of OCD genetic risk.