Binge-eating disorder, a new official diagnosis in DSM-5 (
1), is a serious public health problem (
2) with substantial social and economic costs (
3). Binge-eating disorder is defined by recurrent binge eating (eating unusually large amounts of food while experiencing loss of control) and marked distress without inappropriate weight-compensatory behaviors (
1). Binge-eating disorder is prevalent, is associated with elevated rates of psychiatric and medical disorders and with psychosocial impairments (
2,
4,
5), and is predictive of future medical conditions (
2,
6). While binge-eating disorder is associated strongly with obesity (
2,
4), this psychiatric disorder has behavioral, psychopathological, and neurobiological features distinct from the medical diagnosis of obesity (
7). Despite high levels of morbidity, binge-eating disorder remains underrecognized, and most people with binge-eating disorder go untreated (
2,
8).
Controlled treatment research has identified certain psychological and pharmacological treatments with efficacy for binge-eating disorder (
9,
10). Cognitive-behavioral therapy (CBT) and interpersonal psychotherapy (IPT) have empirical support for binge-eating disorder, generally resulting in 50% remission rates and durable therapeutic benefits through 24-month follow-ups (
11). These “specialist” treatments, however, are neither frequently sought nor widely available (
8), and they fail to produce weight loss (
11,
12). Growing evidence suggests that behavioral weight loss therapy (BWL), a “generalist” and disseminable behavioral treatment (
11), produces binge-eating outcomes in binge-eating disorder that are comparable to CBT and IPT but with the advantage of significant weight loss (
11–
13). BWL trials have reported binge-eating remission rates ranging from 38% to 74% and percent weight loss ranging from 2.6% to 5.1% (
11–
13).
The sole pharmacological treatment for binge-eating disorder approved by the U.S. Food and Drug Administration (FDA) is lisdexamfetamine, which results in binge-eating abstinence rates of 32%–40% (
14,
15). Lisdexamfetamine, however, is contraindicated for individuals with a history of substance misuse, and a “limitation of use” section in its labeling notes that it is not indicated for weight loss and that its safety and effectiveness for the treatment of obesity have not been established. Several other medications have yielded statistically significant reductions in binge eating (
10), but only topiramate has reliably reduced both binge eating and weight (
16), used alone and when combined with psychological treatments (
17,
18). Unfortunately, topiramate has limited tolerability and high discontinuation rates (
10).
Many patients do not benefit sufficiently even when they receive best-available treatments for binge-eating disorder (
9,
10). The strong association between binge-eating disorder and obesity, along with patients’ treatment goals, suggests that focusing solely on either binge eating or on weight is a false dichotomy that fails to meet patients’ preferences and medical needs (
19). To date, achieving weight loss in patients with binge-eating disorder and obesity has been difficult, and BWL is the sole psychological approach that has reliably produced weight loss alongside reduced binge eating (
13). Weight losses with existing treatments for binge-eating disorder are often insufficient (
18) and are frequently less than those reported with treatments for obesity without binge-eating disorder (
20). Thus, identifying effective pharmacological and combination approaches for reducing both binge eating and weight in binge-eating disorder remains a pressing need (
18).
Although there are several FDA-approved weight loss medications, no centrally acting agents have been tested alone and combined with psychological treatments for binge-eating disorder (
18). Orlistat, a lipase inhibitor, was found to significantly enhance weight loss, albeit only modestly, and effects on binge eating were nonsignificant (
21). One FDA-approved obesity medication, a naltrexone-bupropion combination (
22), is a logical treatment to consider for binge-eating disorder because its putative mechanisms are relevant for both binge eating and obesity. Naltrexone-bupropion has hypothesized effects in regulating food intake and weight based on leptin’s mechanisms of action (
23). Leptin’s excitatory effects on pro-opiomelanocortin (POMC) neurons in the hypothalamic melanocortin system produce anorectic effects (
24). Stimulated POMC signaling decreases food intake and increases energy expenditure but is inhibited by endogenous feedback (
24). The rationale for this medication combination is to stimulate POMC neurons (bupropion) and to block endogenous feedback that inhibits POMC activity (naltrexone) (
23,
25). Trials have found naltrexone-bupropion effective for obesity (
25,
26), leading to FDA approval (
22), and one trial found that it significantly enhanced BWL outcomes for obesity (
27). A pilot study reported that naltrexone-bupropion was well tolerated in patients with binge-eating disorder, and a greater proportion of patients in the active treatment group attained weight loss relative to the placebo group (
28).
The present study is a randomized double-blind placebo-controlled trial designed to test the effectiveness of BWL and naltrexone-bupropion, alone and in combination, for binge-eating disorder with comorbid obesity.
Methods
This single-site randomized controlled trial protocol was approved by the Yale institutional review board and followed a data safety and monitoring plan with a physician safety officer. Participants provided written informed consent.
Participants
Participants were recruited through advertisements. Eligibility criteria included meeting DSM-5 criteria for binge-eating disorder, age between 18 and 70 years, and a body mass index (BMI) between 30.0 and 50.0 (or ≥27.0 with obesity-related comorbidity). Minimal exclusion criteria were used, comprising clinical issues that, regardless of setting, would dictate a need for alternative treatment or represent contraindications to naltrexone-bupropion. Exclusionary criteria included concurrent treatment for eating or weight disorders, taking contraindicated medications (e.g., opioids), uncontrolled medical conditions or contraindications to naltrexone-bupropion (e.g., seizure history, bulimia nervosa or anorexia nervosa history, cardiovascular disease, psychosis, bipolar disorder, systolic blood pressure >160 mmHg, diastolic blood pressure >100 mmHg, or heart rate >100 beats/minute), and pregnancy or breastfeeding.
Assessments
Assessment procedures were performed by doctoral-level research clinicians who were monitored throughout the study. The primary outcomes (binge eating, measured weight) and secondary outcomes were assessed using a battery of established interviews, self-report measures, and laboratory tests selected to assess specific eating and weight disorder constructs and associated psychological and metabolic variables.
The Eating Disorder Examination interview (16th ed.) (
29) was administered to diagnose binge-eating disorder and assess binge-eating frequency and eating disorder psychopathology at baseline and posttreatment assessments. This interview, often used as a primary measure in eating disorder randomized controlled trials (
9), has good interrater and test-retest reliability in binge-eating disorder (
30); the global score (for which Cronbach’s alpha=0.81) reflects eating disorder psychopathology. In this study, interrater reliability (N=50) of the Eating Disorder Examination interview was excellent; intraclass correlation coefficients were 0.91 (95% CI=0.84, 0.95) for binge-eating frequency and 0.98 (95% CI=0.97, 0.99) for the global score.
Weight and height were measured at baseline, and weight was measured at the monthly and posttreatment assessments. Fasting cholesterol levels (total, HDL, and LDL) and glycated hemoglobin A1c (HbA1c) levels were obtained at the baseline and posttreatment assessments.
A battery of self-report measures was completed at the baseline, monthly, and posttreatment assessments. The Eating Disorder Examination Questionnaire, which has good test-retest reliability (
31), was used to obtain past-28-day binge-eating frequency data. The Beck Depression Inventory–II (Cronbach’s alpha=0.91) is a well-established measure of depression symptoms and levels (
32). The Three-Factor Eating Questionnaire (alpha=0.72) measures eating behaviors (cognitive restraint, disinhibition, and hunger); it is validated and shows differential responses across treatments consistent with putative mechanisms (
33). The Food Craving Inventory (alpha=0.90) is a measure of cravings for specific foods and is validated in binge-eating disorder (
34). The Power of Food Scale (alpha=0.93) assesses psychological drive to consume palatable foods; it measures appetite (not consumption) for palatable foods and is validated (
35).
Randomization
Participants were randomized to one of four treatments, in a balanced 2×2 factorial design, for 16 weeks: placebo, naltrexone-bupropion, BWL+placebo, or BWL+naltrexone-bupropion. Randomization, without stratification, assigned participants to the four treatments in blocks of eight (to obviate secular trends and to ensure approximately equal proportions). A biostatistician developed the randomization schedule, which was concealed prior to each randomization. Medication was double-blind; naltrexone-bupropion and placebo were prepared in capsules and matched in appearance by the Yale Investigational Drug Service. Randomization assignment to BWL conditions was kept blinded from participants until treatment started. Assessors of posttreatment outcomes were blinded to whether participants received BWL in addition to the (double-blind) medication. Participants were reminded before each assessment not to disclose any details about treatments during meetings with assessors.
Treatments
Behavioral weight loss therapy (BWL).
BWL followed the protocol originally developed and refined for obesity trials (
36) and since adapted for binge-eating disorder (
12,
13). BWL was delivered in individual 45-minute sessions following the manualized protocol. Participants were given patient-version manuals covering all the BWL information and components. Weekly homework assignments were keyed to specific material to reinforce learning and using behavioral techniques. BWL focuses on gradual behavioral lifestyle changes, including moderate caloric decreases (with a goal of approximately 1500 kcal/day), improved nutrition quality (<30% fat), and moderate physical activity (30 minutes five times weekly). Behavioral techniques include goal setting, monitoring food intake and physical activity, stimulus control to achieve and maintain the lifestyle changes, and problem-solving skills to overcome challenges.
BWL was delivered by 11 research clinicians with programmatic interests in eating disorders and obesity; six were clinical psychology graduate students (with a mean of 4.3 years of graduate education) and five were postdoctoral psychologists (with a mean of 8.0 years of years graduate education). Clinicians received intensive training in the manualized protocols and were supervised weekly (including reviews of recorded sessions) by the investigators to monitor quality and adherence. Supervision included review of the structure, process, and content of sessions to ensure fidelity and to prevent drift, per previous trials (
12,
13).
Medication (naltrexone-bupropion or placebo).
The naltrexone-bupropion combination comprised 32 mg/day of sustained-release naltrexone and 360 mg/day of sustained-release bupropion; two tablets were taken twice daily, each containing 8 mg of naltrexone and 90 mg of bupropion. Placebo was given in capsules matched in appearance and frequency. Naltrexone-bupropion dosing began with one-quarter of the full dose and was increased weekly until full dose was achieved by the fourth week (
26,
27). If patients developed intolerable side effects, the study physician reduced dosing to achieve tolerability; if patients experienced adverse events or could not tolerate the medication, they were withdrawn from medication.
Two faculty-level study physicians delivered the pharmacotherapy, focused on medication management (addressing adherence, safety, and side effects). Additional psychotherapeutic interventions were proscribed. Medication adherence and detailed side effect and safety checklists were performed monthly. Monthly medication refills were accompanied by re-review of medication adherence and dosing schedules, and pill bottles were returned for pill counts at the posttreatment assessment.
Statistical Analysis
Sample size was based on power calculations using data from randomized controlled trials testing BWL for binge-eating disorder (
11,
12), placebo for binge-eating disorder (
14,
15), and naltrexone-bupropion for weight loss (
25,
26). Although some reported effect sizes have been in the medium to large ranges, we conservatively powered this trial to detect medium effects after considering clinically meaningful effect sizes and performing sensitivity analyses for different outcomes. A sample of size of 140, allocated to four treatment conditions, yielded >80% power for medium effect sizes (Cohen’s f=0.25) for main effects for BWL and naltrexone-bupropion treatments and for interaction effects between BWL and naltrexone-bupropion at a two-sided alpha of 0.05, even after assuming 20% attrition.
Analyses to compare treatments were all intention-to-treat and were performed for all randomized patients who attended the first treatment session. The two co–primary outcome variables were binge eating and weight loss, both analyzed using complementary approaches. Binge eating was analyzed as continuous and categorical outcomes (i.e., monthly frequency and remission, respectively). “Remission” from binge eating was defined as zero episodes during the previous 28 days (on the Eating Disorder Examination interview), with any missing data imputed as nonremission. Weight loss (measured) was analyzed as continuous and categorical outcomes (i.e., percent weight loss and attaining 5% weight loss, respectively). The 5% weight loss category was based on measured weight loss from baseline, with missing data imputed as failure to have achieved 5% weight loss; this is a common outcome measure in obesity trials (
26,
27,
36) because it is associated with clinical benefits (
37). Secondary treatment outcomes were eating disorder psychopathology (Eating Disorder Examination interview global score), depression (Beck Depression Inventory–II), eating behaviors (Three-Factor Eating Questionnaire, Food Craving Inventory, Power of Food Scale), and cardiometabolic and endocrine variables (cholesterol and HbA
1c levels).
For analyses of continuous measures, intention-to-treat analyses used all available data in mixed models without imputation. Variables not conforming to normality were log-transformed prior to analysis. Mixed-effects models (
38) were fitted with fixed factors including BWL treatment (yes vs. no), medication (naltrexone-bupropion vs. placebo), time (all relevant time points from among the baseline, month 1, month 2, month 3, and posttreatment assessments), and all possible interactions. In each model, we considered different error structures and selected the best-fitting structure using the Schwarz’s Bayesian criterion. Focused comparisons of least-square means (effect slices) were used to explain significant effects in the models. Statistical testing was performed at the 0.05 significance level.
For categorical outcomes (e.g., binge-eating remission, 5% weight loss), logistic regression tested the outcomes at the posttreatment assessment. The independent variables were the two treatments (BWL and medication, each at two levels) and their interaction. Odds ratios and 95% confidence intervals were used to explain significant effects in the models.
We explored whether time of measurement (before or during the COVID-19 pandemic; in-person vs. remote visit) affected the results by including an indicator for timing of measurement as a covariate. The results did not change substantively, and therefore the final models are not adjusted for changes in visits during the pandemic.
Results
The study was conducted from February 2017 to February 2021.
Figure 1 summarizes participant flow throughout the study. Of the 2,648 respondents screened, 289 consented and were evaluated for eligibility, and 136 were randomized and attended the baseline assessment.
Table 1 summarizes the participants’ sociodemographic characteristics, and
Table 2 summarizes their clinical characteristics. The participants’ mean age was 46.5 years (SD=12.2), and the sample’s mean BMI was 37.1 (SD=4.9); 81.6% (N=111) were female, 84.6% (N=115) attended or finished college, and 77.9% (N=106) were White.
Thirty-four participants were randomized to received placebo, 32 to naltrexone-bupropion, 35 to BWL+placebo, and 35 to BWL+naltrexone-bupropion. Posttreatment assessments were obtained for 82.4% of participants; rates did not differ significantly across treatments.
Primary Outcomes
Table 2 presents descriptive statistics for the co–primary outcomes (binge eating and weight loss) and
Figures 2 and
3 summarize analyses for continuous and categorical variables; Table S1 in the
online supplement shows the test statistics and p values for all the main and interaction effects in the mixed models for the co–primary continuous outcomes.
Figure 2A illustrates intention-to-treat binge-eating remission rates at the posttreatment assessment by treatment condition. Remission rates were 17.7% for the placebo group, 31.3% for the naltrexone-bupropion group, 37.1% for the BWL+placebo group, and 57.1% for the BWL+naltrexone-bupropion group. Logistic regression revealed significant main effects of BWL (χ
2=7.45, df=1, p=0.006) and of medication (χ
2=4.18, df=1, p=0.04) but no significant BWL-by-medication interaction (χ
2=0.007, df=1, p=0.94). BWL (compared with no BWL) was associated with nearly three times higher odds of remission (odds ratio=2.84, 95% CI=1.34, 6.03), whereas naltrexone-bupropion (compared with placebo) was associated with roughly two times higher odds of remission (odds ratio=2.19, 95% CI=1.03, 4.63).
Mixed-model analyses of binge-eating frequency (episodes during the past month) at the posttreatment assessment (Eating Disorder Examination interview) revealed a significant interaction between BWL and time (F=24.34, df=1, 111, p<0.0001) and a significant main effect of time (F=239.39, df=1, 111, p<0.0001). Medication effects were not statistically significant (see Table S1 in the
online supplement for full results). Binge-eating frequency decreased significantly from baseline to posttreatment assessment in the BWL groups (F=16.01, df=1, 110, p<0.0001) but not in the no-BWL groups (F=3.30, df=1, 132, p=0.07).
Figure 2B summarizes frequency of binge eating (during the past month), assessed monthly throughout the course of treatment. Mixed models revealed significant interactions between BWL and time (F=5.99, df=4, 407, p<0.0001) and between medication and time (F=2.57, df=4, 407, p=0.04), but the three-way interaction was not significant (p=0.37). The main effect of time was also significant (F=92.84, df=4, 407, p<0.0001). BWL was associated with significantly lower frequency at all postbaseline time points compared with no BWL (p values <0.02), whereas naltrexone-bupropion was associated with significantly lower frequency at months 1 and 2 (p values <0.02) but not at the month 3 or posttreatment assessments (p values >0.16) compared with placebo.
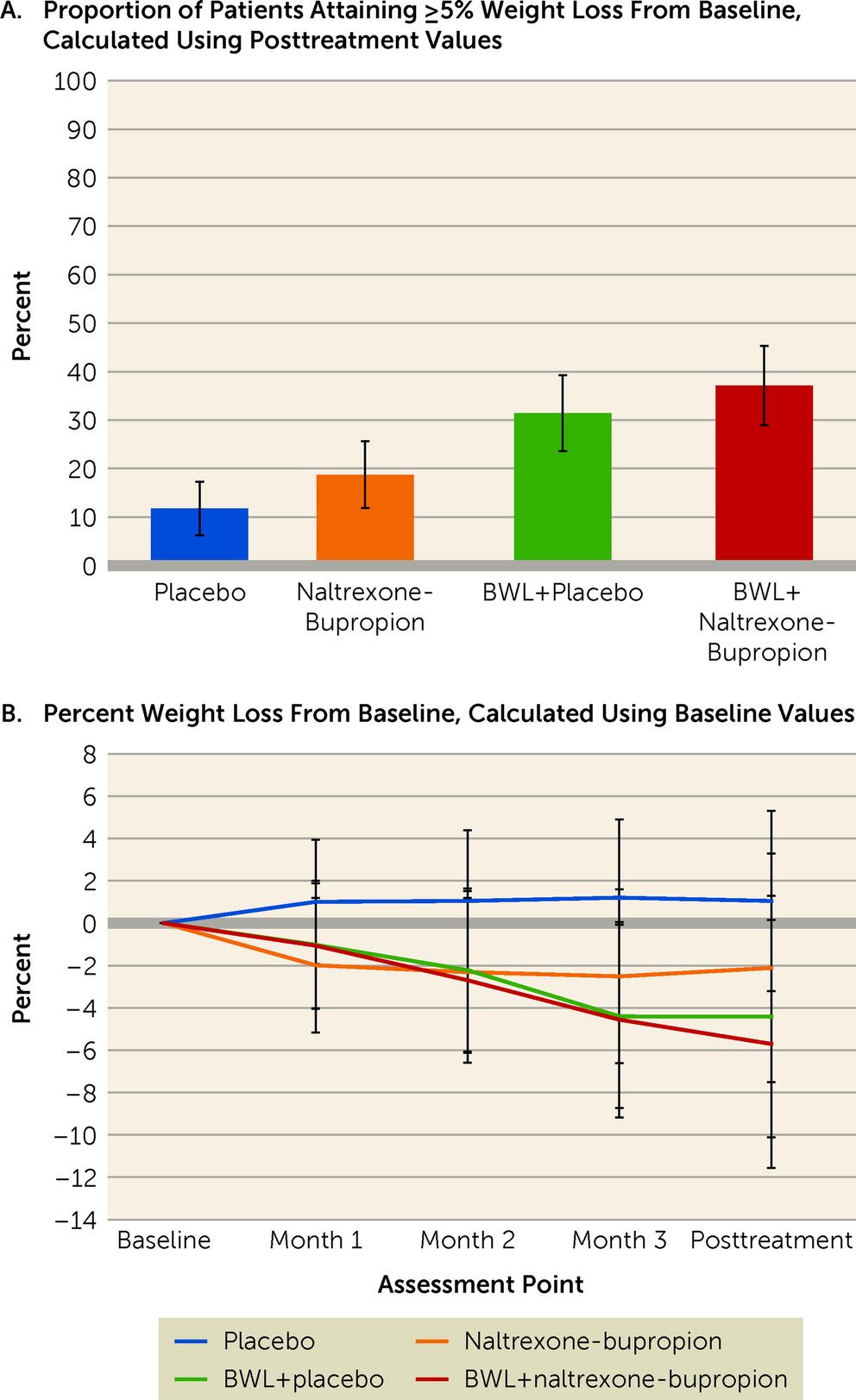
Figure 3A shows intention-to-treat rates of participants who had attained 5% or greater weight loss at the posttreatment assessment, by treatment condition. Rates of participants attaining ≥5% weight loss were 11.8% for the placebo group, 18.8% for the naltrexone-bupropion group, 31.4% for the BWL+placebo group, and 37.1% for the BWL+naltrexone-bupropion group. Logistic regression revealed a significant main effect of BWL (χ
2=6.36, df=1, p=0.01) but no significant main effect of medication (χ
2=0.87, df=1, p=0.35), nor a significant BWL-by-medication interaction (χ
2=0.12, df=1, p=0.73). The odds of attaining ≥5% weight loss were three times higher in the BWL groups than in the no-BWL groups (odds ratio=2.97, 95% CI=1.27, 6.91).
Figure 3B summarizes percent weight loss throughout the course of the treatments, and
Table 2 shows weight values at the baseline and posttreatment assessments, along with changes. Mixed models of percent weight loss revealed a significant interaction between BWL and time (F=10.70, df=3, 111, p<0.0001) and a significant main effect of time (F=12.56, df=3, 111, p<0.0001), but no significant interaction between medication and time (F=0.58, df=3, 111, p=0.63) and no significant three-way interaction (p=0.32). Comparisons between the BWL and no-BWL conditions were significant (p values <0.01) at the posttreatment assessment and at all monthly time points except month 1.
Secondary Outcomes
Table 2 presents descriptive statistics for secondary outcomes of eating disorder psychopathology, depression, eating behaviors, and metabolic variables across treatments.
For eating disorder psychopathology, mixed models of Eating Disorder Examination global scores revealed a significant three-way interaction between BWL, medication, and time (F=9.98, df=4, 114, p=0.002), a significant interaction between BWL and time (F=12.59, df=1, 114, p=0.0006), and a significant main effect of time (F=94.61, df=1, 114, p<0.0001). Significantly greater decreases in eating disorder psychopathology occurred in the BWL conditions than in the no-BWL conditions (p=0.02).
Mixed models of depression scores revealed a significant interaction between BWL and time (F=3.35, df=4, 405, p=0.01) and a significant main effect of time (F=24.53, df=4, 405, p<0.0001). Depression scores decreased significantly more in the BWL conditions than in the no-BWL conditions, with the difference statistically significant at later monthly time points and at the posttreatment assessment (p values <0.04).
Mixed models of eating behaviors measured by the Three-Factor Eating Questionnaire (restraint, disinhibition, and hunger scales) revealed consistent patterns of significantly greater improvements with BWL than no BWL. For each of the three scales, there were significant interactions between BWL and time (F=23.87, df=2, 225, p<0.0001; F=17.66, df=2, 234, p<0.0001; and F=9.02, df=2, 226, p=0.0002, respectively) and significant main effects of time (F=22.45, df=2, 225, p<0.0001; F=64.52, df=2, 234, p<0.0001; and F=53.00, df=2, 226, p<0.0001, respectively), but nonsignificant medication effects. Mixed models of other eating behaviors (craving [Food Craving Inventory] and drive to consume palatable foods [Power of Food Scale]) also revealed consistent patterns of significantly greater improvements with BWL than without BWL. There were significant interactions between BWL and time (F=5.12, df=2, 219, p=0.007, and F=18.61, df=2, 225, p<0.0001, respectively, for craving and drive to consume foods) and significant main effects of time (F=72.31, df=2, 219, p<0.0001; F=97.29, df=2, 225, p<0.0001), but nonsignificant medication effects.
Mixed models of total cholesterol revealed a significant interaction between BWL and time (F=4.66, df=1, 107, p=0.03) but not between medication by time; mixed models for HDL and LDL, however, did not reveal significant effects. Mixed models of HbA1c revealed a significant interaction between BWL and time (F=5.85, df=1, 106, p=0.02) and a significant main effect of time (F=15.51, df=1, 106, p=0.0001), but there were no significant medication effects.
Discussion
In this study of adults with binge-eating disorder and obesity, BWL and naltrexone-bupropion were associated with significant improvements, with a consistent pattern of BWL being superior to no BWL. The co–primary outcomes were binge eating (i.e., attaining remission and frequency) and weight loss (i.e., attaining 5% weight loss and percent loss). For binge-eating remission, BWL was significantly superior to no BWL, and naltrexone-bupropion was significantly superior to placebo, but there was no significant interaction between BWL and medication. For binge-eating frequency, analyses using complementary measures revealed that BWL was significantly superior to no BWL but that naltrexone-bupropion was not significantly superior to placebo, and the interaction between BWL and medication was not significant. For weight loss, analyses of rates of attaining 5% weight loss and of percent weight loss both converged, revealing that BWL was significantly superior to no BWL but that naltrexone-bupropion was not superior to placebo, and there was no significant interaction between BWL and medication. Analyses of secondary measures converged, indicating that BWL, but not naltrexone-bupropion, was associated with significant improvements across broad outcomes (eating disorder psychopathology, depression, eating behaviors, cholesterol levels, and HbA1c level).
The findings provide further support for the effectiveness of BWL for binge-eating disorder, extending previous studies of BWL (
11–
13) in important ways. First, the superiority of BWL in this study supports the “specificity” of the effectiveness of BWL (i.e., statistical superiority over a credible pharmacologic treatment) (
9). Second, the effectiveness of BWL for binge-eating disorder was observed across broad outcomes reflecting eating, psychological, and metabolic clinical domains. Finally, the significant weight loss findings (means of −4.4% for BWL+placebo and −5.7% for BWL+naltrexone-bupropion, with 31.4% and 37.1% of patients, respectively, attaining 5% or greater weight loss) closely approximate the findings reported in recent trials for BWL without pharmacotherapy for binge-eating disorder (
11–
13). These findings are encouraging given the well-known difficulty in producing weight loss in patients with binge-eating disorder and comorbid obesity (
9,
18,
20). In comparing our 16-week findings to those of a similar, albeit substantially longer (56-week), trial for obesity without binge-eating disorder (
27), the mean 4.4% weight loss for BWL+placebo approximates their 4.9% weight loss, whereas the mean 5.7% weight loss for BWL+naltrexone-bupropion was less than their 7.8% weight loss (
27).
The study findings provide support for the potential effectiveness of naltrexone-bupropion for binge-eating disorder. The binge-eating remission rate for the naltrexone-bupropion group (31.3%), which had roughly two times higher odds of remission than the placebo group, approximated the 36%–40% rates reported for lisdexamfetamine (
15), the sole FDA-approved medication for binge-eating disorder. Unlike lisdexamfetamine, which is not indicated for obesity, naltrexone-bupropion is FDA-approved for obesity, and this study employed fewer exclusionary criteria than lisdexamfetamine trials (
14,
15). In terms of weight loss, however, naltrexone-bupropion did not differ significantly from placebo, and the observed 2.1% weight loss was lower than the 6.1% reported for obesity without binge-eating disorder (
26).
Analyses revealed nonsignificant interaction effects between BWL and naltrexone-bupropion, which converges with previous binge-eating disorder studies testing other medications combined with various psychotherapies (
18), except topiramate (
17). Future research should include comparative tests of different medications, and of psychological versus pharmacological approaches, designed with a priori tests of moderators of outcomes.
Study strengths include manualized behavior therapy and pharmacotherapy delivered by trained and monitored psychologists and physicians, independent assessments using well-validated measures, minimal exclusionary criteria to enhance generalizability, and good retention. Several study limitations are noteworthy. The generalizability of findings to different settings or persons with different sociodemographic and clinical characteristics is uncertain. The sample size had limited power to detect smaller-magnitude main or interaction effects of treatments. We did not include a BWL-only condition (without placebo). Longer-term outcomes of these acute findings are unknown. Whether variations in clinicians’ adherence or patients’ adherence to the behavioral and pharmacologic interventions moderate the outcomes is unknown. With these strengths and limitations as context, we conclude that BWL and naltrexone-bupropion were associated with significant improvements in patients with binge-eating disorder and obesity, with BWL demonstrating superior improvements.