The pathology of diffuse axonal injury (DAI), a major form of traumatic brain injury (TBI), is characterized histologically by Wallerian-type axonal degeneration in the parasagittal white matter, corpus callosum (CC), and dorsal upper brainstem, resulting from shearing forces caused by sudden acceleration, deceleration, or rotation of the brain.
1 Brain damage in DAI was originally thought to occur diffusely in the white matter.
2Advances in neuroimaging techniques have allowed the examination of structural brain alterations in patients with DAI. For example, voxel-based morphometry (VBM)
3 has revealed the presence of brain-volume reductions in the frontal and temporal lobes, the cingulate, the CC, and in subcortical regions such as the thalamus and caudate in patients with DAI.
4–6 These observations suggest that brain alterations in DAI are not restricted to the white matter, and that other specific brain regions are susceptible to injury as well. In addition, diffusion tensor imaging (DTI) studies have shown reductions in fractional anisotropy (FA), a measure of alterations in white matter microstructure, in various areas in patients with DAI, including the CC, internal/external capsule, and the superior/inferior longitudinal fascicles. Among these regions, the CC is thought to be especially vulnerable because robust FA reductions have been reported in this area.
7–9Symptoms after TBI primarily result from dysfunction in the damaged brain regions. Impairments in several cognitive domains are common after TBI, including memory and executive functions
10 and the speed of information processing. Among these cognitive domains, impaired information processing speed is one of the most common deficits in patients with TBI.
11 Information processing speed is a measure of the efficiency of cognitive function and is assessed using timed tests that challenge simple cognitive operations.
12 This ability is a fundamental component of many cognitive functions.
13 Studies in people with declining information processing speed, such as occurs in normal aging and some neuropsychiatric diseases, have suggested that white matter pathology is the neural basis of their impairments.
14,15The relationships between cognitive impairments and affected brain regions have been investigated in TBI. However, only a few studies have reported an association between neuropsychological performance and brain alterations in DAI. For example, volume reductions in gray matter structures were found to correlate with poor attention and memory deficits.
16 In addition, studies have reported an association between white matter alterations (e.g., volume and microstructure) and cognitive functioning.
17,18 However, to our knowledge, no study has comprehensively investigated the relationships between MRI measures derived from multiple neuroimaging modalities and cognitive impairments in DAI.
We hypothesized that a neural network comprising the CC may be associated with the cognitive impairments observed in DAI. This study aimed to assess structural brain damage in patients with chronic DAI and to examine the relationship between brain damage and cognitive impairments. We utilized VBM and DTI, along with neuropsychological assessments, to evaluate potential associations.
Discussion
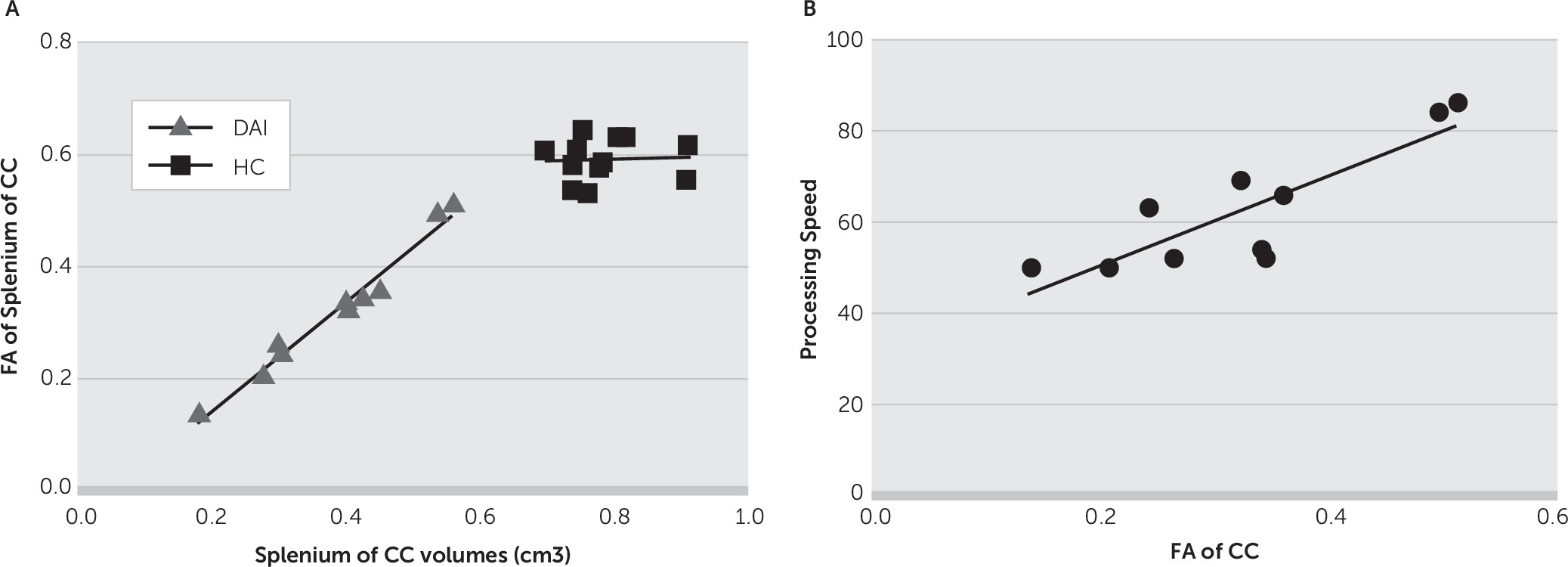
In this study, we investigated the relationship between brain alterations and cognitive impairments in patients with DAI using a combination of multiple structural imaging data. We found reductions in volume and white matter microstructure in the CC in the DAI group. These parameters were correlated with each other; patients with DAI with lower CC volumes showed greater reductions in CC FA values. In the neuropsychological tests, the DAI group showed impairments in all IQs and IQ index scores. The main finding was that impairment in processing speed in DAI correlated with structural alterations in the splenium of the CC.
Neuropsychological assessment revealed that patients with DAI exhibited reductions in all IQs and IQ index scores. Among the IQ index scores, only the processing speed was >2 SD below the standard mean. Processing speed has been most frequently investigated in participants with TBI, and impairments in this cognitive domain have been repeatedly reported.
24–27 However, the injury types in the participants in the previous studies were heterogeneous; they included participants with various lesions. Our results suggest that if the injury type is specified as DAI, cognitive impairment is likely to be most prominent in the domain of processing speed. In this study, we used the IQ index score from the WAIS-III to measure processing speed. This method is one of several ways to measure processing speed. It is relatively complex, using the summed score from the digit symbol and symbol search subtests. Thus, in contrast with a simple task using reaction time, this index reflects abilities in working memory, executive functions, and sensory and psychomotor functions, in addition to processing speed per se.
12 Furthermore, this index is specific to the visual modality. To investigate impairment in processing speed in patients with DAI in more detail, simple tasks (e.g., Part A of the Trail Making Test) or tasks using other modalities (e.g., Paced Auditory Serial Addition Test) are needed.
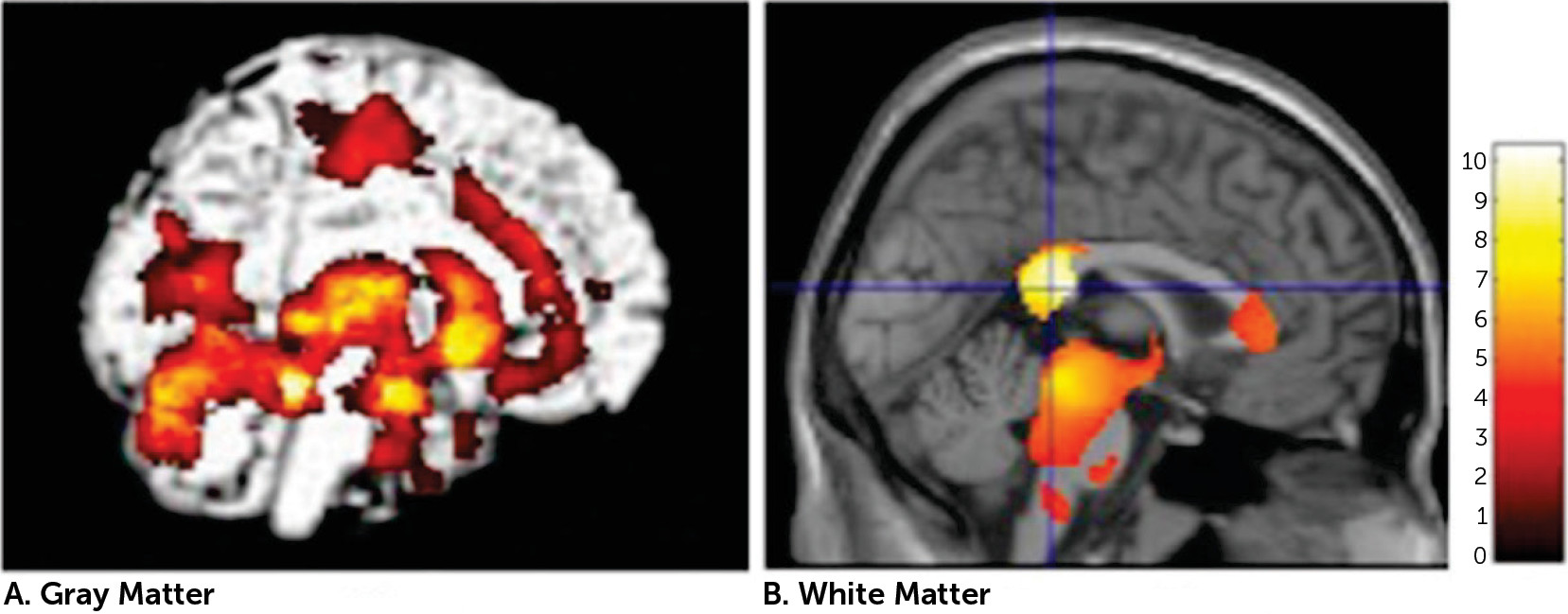
VBM analysis showed volume reductions in widespread regions, including the CC, in patients with DAI. These findings are largely in accordance with earlier VBM studies that showed changes in a variety of central subcortical areas.
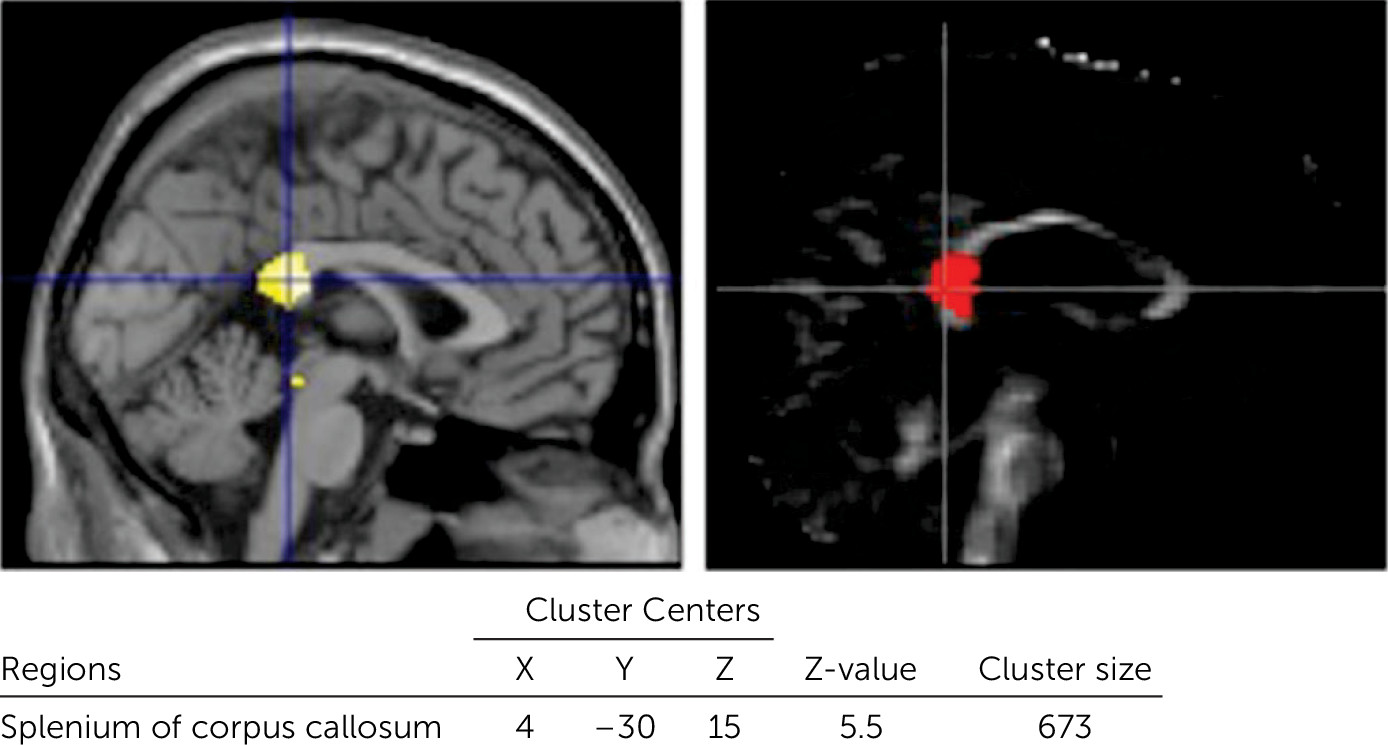
4–6,28 Regional volume reductions were most prominent in the splenium of the CC. Indeed, the CC is a region that is particularly vulnerable to shearing forces, or rotation of the brain due to trauma,
2 and our result is consistent with previous studies.
8,29,30The patients with DAI showed lower FA, a measure of alterations in white matter microstructure, in the CC compared with controls. The FA findings were largely in accord with previous DTI studies in TBI.
31–33 Axon damage is the major pathologic feature associated with reduced white matter microstructure after TBI.
34 Our results revealed that the degree of reduction in FA value and the extent of volume reduction were strongly intercorrelated. This suggests that the remaining fibers are more severely damaged when the volume reduction is more severe. In mice, demyelination, remyelination, and excessive myelin are observed during white matter degeneration and recovery after TBI.
35 Longitudinal studies are needed to elucidate the mechanisms underlying the strong relationship between FA values and fiber volumes in the CC observed in our study.
Significant correlations were found between the processing speed score in the WAIS-III and each of the MRI measures in the splenium of the CC. Similar relationships between FA in the splenium and processing speed were reported in children with DAI.
36 Furthermore, a reduction in white matter microstructure in the CC appears to contribute to the age-related loss in cognitive processing speed.
37 These observations, together with our present findings, suggest that CC pathological changes at least partially underlie the impairment in cognitive processing speed. Anatomically, the splenium of the CC contains pathways that connect the parieto-occipital regions of the two hemispheres.
38 These regions are critical for visuospatial and attentional processes.
39 Indeed, a functional MRI study showed that coordinated activity across multiple networks, including the parietal cortex, is essential for optimal cognitive processing speed.
40 Thus, the disruption of these pathways may contribute to cognitive decline in patients with DAI by interrupting communication between critical cortical structures.
Similar to the severity and duration of loss of consciousness at onset, PTA serves as a marker of injury severity in TBI. However, we found no significant relationships between PTA and each of the MRI measures in the patients. The lack of a significant relationship may be attributable to the fact that our patient sample was in the chronic stage. This suggests that CC pathology in the chronic stage may not reflect injury severity in the acute stage. However, the clinical markers of injury severity (i.e., severity and duration of loss of consciousness at onset, and PTA) may be associated with pathology in other brain regions, such as the brainstem.
This study had several limitations. First, our sample size was small and did not include patients with mild TBI. Thus, this study should be regarded as preliminary. However, despite the small sample size, our results were statistically robust and agree with other clinical observations. Second, we focused on patients with chronic DAI. Consequently, we cannot generalize our findings to patients in the acute or subacute stage. Third, the premorbid IQ and educational level of two patients was low, although the mean premorbid IQ of the patient group as a whole was within the normal range. Because these patients were not able to finish high school because of brain injury, attributing the low premorbid IQ and educational level to the brain injury, and not to any preexisting low IQ, is reasonable. Fourth, because the neuropsychological assessment was conducted using only using the WAIS-III, we may not have assessed all aspects of the cognitive impairments. In addition, we extracted the IQ index score as a measure of processing speed. The processing index score of the WAIS-III is a complex measure using a score summed from a group of subtests. Thus, we cannot identify which aspects of processing speed are impaired. Further studies are needed using comprehensive and detailed tests to assess neuropsychological disturbances in TBI. We focused on structural changes in this study, although functional abnormalities are also found in the brains of patients with DAI. Thus, future studies combining structural and functional neuroimaging techniques are needed to clarify the neuropathological basis of cognitive impairment in patients with DAI.