Insurance coverage for prescription drugs changed from state Medicaid programs to Medicare Part D plans on January 1, 2006, for all beneficiaries enrolled in both programs (“dual beneficiaries”). Dual beneficiaries are among the most severely ill beneficiaries in the Medicaid and Medicare programs (
1), and one-third of them, ages 19 to 64, have a diagnosed serious mental illness (
2,
3). Federal regulations for Part D required inclusion of most psychiatric medications on plan formularies and limited copayment requirements for dual beneficiaries (
4,
5). However, plans may apply utilization management policies to their formulary medications, including prior authorization and step therapy (in which a medication is available only after failure of a plan-preferred medication) (
4). Among adults with bipolar disorder, transitions to more restrictive psychiatric drug utilization management have been significantly associated with reduced initiations and use of recommended pharmacotherapy (
6,
7) and increased treatment discontinuation (
6). Discontinued or delayed use of antimanic medication is in turn associated with increased emergency department use (
8) and health care costs (
9). Medicaid programs have frequently applied prior-authorization and other utilization management strategies to manage the use of antipsychotic, anticonvulsant, and antidepressant medications (
10,
11). It is unclear whether, or to what extent, utilization management policies of Part D plans relaxed or tightened psychiatric medication management relative to those of Medicaid programs.
The evidence on the effects of the transition from Medicaid to Medicare Part D coverage on dual beneficiaries’ receipt of medication for serious mental illness is limited to three observational studies that relied on psychiatrist or patient self-report to define insurance status and prescription drug use. In a 2006 national survey, psychiatrists reported frequent problems with medication access among dual-beneficiary patients during the first year of Part D implementation; however, no comparative information was available on patient experiences before the transition (
12). Research that has compared psychiatric medication use before and after Part D implementation among dual beneficiaries found no change in patients’ self-reported use of antidepressant and antipsychotic medications (
13,
14), but the sample sizes were too small to construct diagnosis-based cohorts to assess use of psychiatric medication relative to recommended use. No published research has assessed dual beneficiaries’ use of other health care services that may signal suboptimal pharmacotherapy for serious mental illness.
This quasi-experimental study evaluated the effects of the initial transition to Part D coverage on receipt of guideline-concordant pharmacotherapy and emergency department use in the first two years after the implementation of Part D for a clinically defined cohort, dual beneficiaries with a diagnosis of bipolar I disorder.
Methods
We used an interrupted–time-series design, which is the strongest quasi-experimental design and requires a discrete intervention, a sufficient number of observation points before and after the intervention, and the absence of a concurrent event that might confound the relationship between intervention and outcome (
15). This design does not require the inclusion of patient covariates in the analytic model if the composition of the study population is stable, as it was in this study. The discrete intervention was the transition from Medicaid to Part D coverage on January 1, 2006.
The University of Wisconsin—Madison Institutional Review Board determined that this study was exempt (protocol 2012-0548).
Data
We merged Medicaid enrollment, Medicare enrollment, and Medicare medical claims data for a 5% national random sample of Medicare beneficiaries from the period 2004–2007. For this sample, we additionally merged Medicare Part D claims data for 2006–2007 and pharmaceutical and medical claims data for 2004–2005 from the Medicaid Analytic Extract files. Enrollment files included dates of enrollment and beneficiary demographic information. To link data across programs, we matched Social Security number, name, and date of birth. Medical claims included the service type, service dates, and diagnoses. Pharmacy claims included the product, fill date, and the number of days’ supply dispensed. These data have demonstrated reliability for research purposes (
16–
18). We excluded beneficiaries residing in Ohio and Louisiana due to data anomalies and excluded those in Arizona because all beneficiaries were enrolled in managed care.
Sample
From an initial sample of 42,388 dual beneficiaries with at least one diagnosis of schizophrenia or bipolar I or bipolar II disorder between 2004 and 2007, we excluded individuals with fewer than ten months of continuous fee-for-service dual enrollment in each of the four years to eliminate the possibility that changes in population composition coincident with the Part D transition might bias our results. To ensure that individuals had prescription drug coverage in each year, we excluded beneficiaries who had no prescription drug claim from 2004 to 2007. Health care use data for managed care enrollees were not observable; thus we restricted the cohort to fee-for-service enrollees. Similarly, we excluded individuals with an institutional stay of more than three months because institutional prescription drug use data were not available. Serious mental illness is disproportionately represented in the nonelderly population of dual beneficiaries (
19). Thus we excluded dual beneficiaries over the age of 65.
Consistent with prior work, we required one inpatient or two outpatient diagnoses of bipolar disorder (specifically,
ICD-9 codes 296.0x, 296.1x, 296.4x−296.7x, 296.80–296.82, and 296.89) on different dates of service, with at least one of these diagnoses indicating bipolar I disorder (specifically,
ICD-9 codes 296.0x, 296.1x, and 296.4x−296.7x) (
20). We selected enrollees with a bipolar I diagnosis because it is the more severe form of bipolar disorder and pharmacotherapy recommendations are more uniform and clear (
21). We excluded beneficiaries with any diagnosis of schizophrenia. Finally, at least one bipolar disorder diagnosis must have occurred between January and June 2004 to increase the likelihood that bipolar disorder pharmacotherapy would have been appropriate for individuals in each month for which outcomes were assessed. The final analytic sample included 1,431 adults. [Detailed information on the construction of the sample is available online in the
data supplement to this article.]
Outcome measures
Our measures of pharmacotherapy quality were derived from clinical practice guidelines (
22,
23) and U.S. Food and Drug Administration indications. Recommendations for the acute and maintenance phases of bipolar I disorder include continuous treatment with one or more antimanic agents (lithium, carbamazepine, divalproex sodium, lamotrigine, valproate sodium, and valproic acid) or an antipsychotic medication. Therefore, we defined the receipt of these medications as indicators of recommended pharmacotherapy. For patients with a diagnosis of bipolar I disorder, antidepressant medication without a concurrent antimanic medication is contraindicated due to concerns that unopposed antidepressant treatment could worsen the course of bipolar disorder. Thus our indicator of poor-quality treatment was antidepressant monotherapy.
We constructed a binary indicator for receipt of any recommended antimanic agent in a month. We used the medication possession ratio (MPR) to measure Part D–related changes in the level of concordance with evidence-based antimanic pharmacotherapy (
24). The MPR is the number of days of medication that a beneficiary has received divided by the number of days’ supply that the beneficiary should have received if taking the medication as prescribed. We operationalized the recommendation for continuous antimanic pharmacotherapy as the receipt of an antimanic medication sufficient to cover at least 80% of days in a month (
19,
25). We defined receipt of antidepressant monotherapy for seven or more days in the month as a measure of poor quality. In usual care, there may be reasonable, brief delays in prescription fills, and several days of antidepressant monotherapy are unlikely to be of clinical significance.
For all pharmacotherapy measures, the numerator was defined by using the prescription fill date and the number of days of medication supplied. The number of days supplied was apportioned across months. The denominator included the number of days in the month minus the number of days of residence in an acute inpatient facility, because inpatient medication is not ascertainable in claims data (
19,
26). For overlapping fills, we treated drugs of the same generic name and the same National Drug Code (NDC) sequentially, and those with the same generic name and different NDCs concurrently. We treated overlapping fills for more than one generic drug name within the same drug class as concurrent fills.
Our secondary outcomes included the probability and count of emergency department visits for any cause. In addition, we assessed emergency department visits related to a mental disorder or to a substance use disorder. An emergency department visit was considered related to a mental or substance use disorder if the primary or secondary diagnosis on the claim indicated a behavioral health condition as defined by the U.S. Agency for Healthcare Research and Quality (per
ICD-9 codes 290.xx−319.xx; 648.3x, and 648.4x) (
27). The denominator for all emergency department measures was the full analytic sample.
Independent variables included a binary variable for Part D. The value of this variable was set to 0 before the implementation of Part D and set to 1 for the months after its implementation to estimate the potential immediate change in the outcome measure. A second variable, “time after Part D,” represented the number of months after Part D implementation to estimate potential change in trend.
Analyses
Our sample was defined in part by the presence of at least one bipolar disorder diagnosis between January and June 2004, a diagnosis that was observable because the patient had had a health care visit. On the basis of experience in other studies (
6–
8), we expected to observe some decrease in event rates after this diagnosis ascertainment period for outcomes that were dependent on the occurrence of a visit (such as a prescription for an antimanic medication) or that were themselves health care visits (emergency department use). These elevated rates naturally decline to the population’s underlying mean rate of use over time. We conducted sensitivity analyses to explore the duration required to reach steady state by defining a sample that was identical to the study sample except for a later timing of the first observed diagnosis of bipolar disorder (July to December 2004) and assessing study outcomes both before and after the first observed bipolar disorder diagnosis. On the basis of these analyses, we excluded from our models the months during which we required a bipolar diagnosis (January to June 2004) and an additional six months (July to December 2004) to reach steady state.
We used segmented linear regression to estimate cohort-level changes in the outcomes from the pre–Part D period (January 2005 to November 2005) to the post–Part D period (April 2006 to December 2007). This approach estimates a slope for each of the time segments (pre– and post–Part D). We began the Part D period in April 2006 because Part D plans were required to cover prescription fills for nonformulary medications through March 31, 2006. About two-thirds of states implemented for dual beneficiaries some type of transitional drug coverage in January and February of 2006, ranging from one to six weeks in duration (
28). We excluded the four-month phase-in period from December 2005 to March 2006 from our regression models.
The unit of analysis was the person-month. All regression models controlled for a first-order autoregressive correlation structure. From the regression results, we estimated the predicted value of each outcome and its 95% confidence interval (
29) for April 2007, 12 months after the phase-in period. Regression results presented were significant at p<.05 unless otherwise stated. We used two-tailed statistical tests throughout and conducted analyses using Stata, version 12.
Results
Baseline descriptive characteristics
As shown in
Table 1, most of the sample was female. Approximately 67% of beneficiaries were between the ages of 35 and 54, and a large majority was white (89%). The average percentage of beneficiaries who had the recommended antimanic medication available each month was 72%, whereas an average of 62% of beneficiaries had an MPR of at least 80%. The mean percentage receiving antidepressant monotherapy for seven or more days per month was 17%. An average of 12% of beneficiaries had an emergency department visit each month, whereas the mean monthly number of visits among all sample members was .16. Roughly 6% of beneficiaries per month had an emergency department visit with a primary or secondary diagnosis of a mental or substance use disorder, and the mean monthly number of such visits was .07.
Time-series regression results
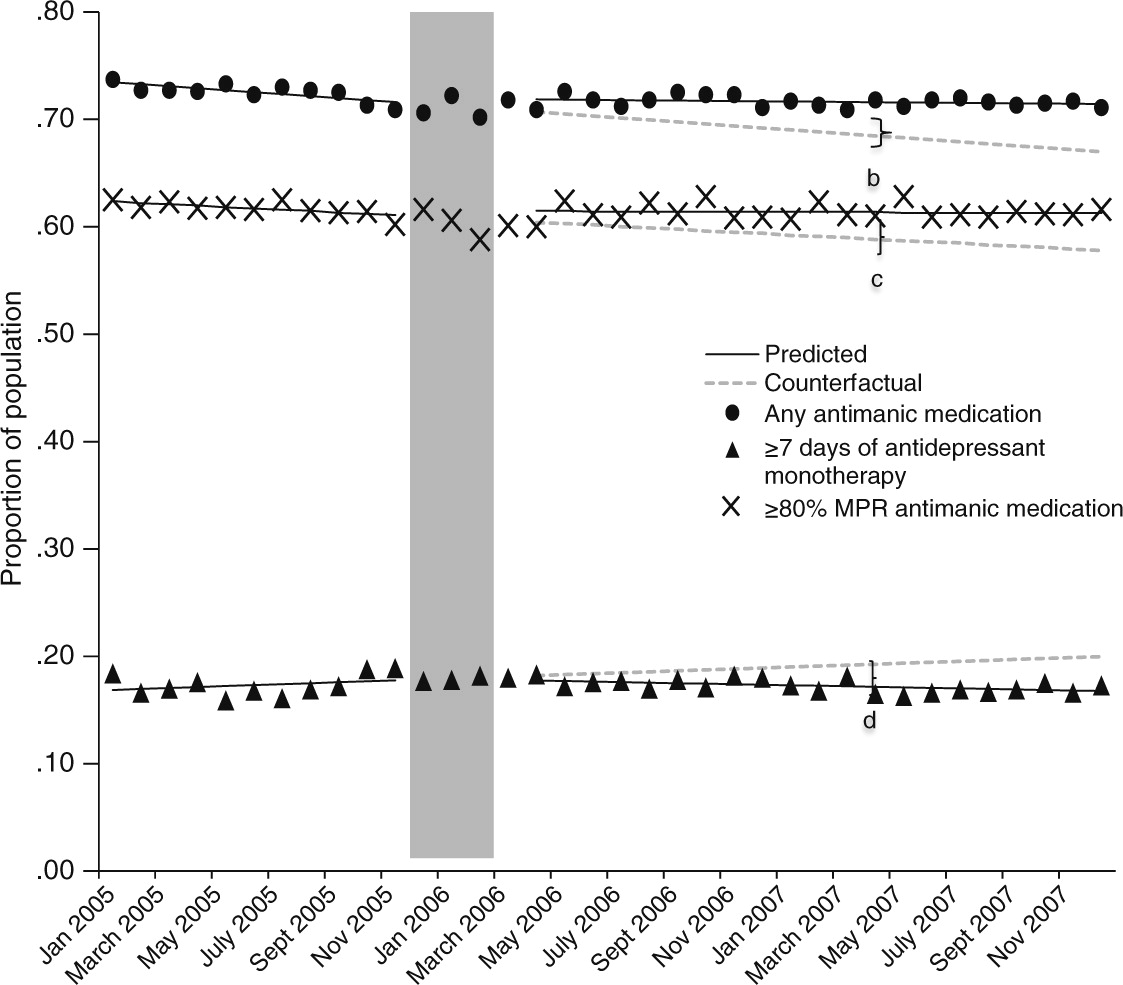
By April 2007, the mean proportion of the population with any recommended use of antimanic medications was an estimated 3.1 percentage points higher than expected in the absence of Part D (
Figure 1 and
Table 2), for a relative increase of 4.6%. The mean monthly proportion of the population that had an MPR of at least 80% for a recommended antimanic medication was declining before implementation of Part D. After the transition, this declining trend leveled off, resulting by April 2007 in a marginally significant absolute increase of 2.5 percentage points (p=.06) in the mean proportion of the population with an MPR ≥80%. The predicted proportion of beneficiaries with seven or more days of antidepressant monotherapy was an estimated 2.1 percentage points lower in April 2007 than expected in the absence of Part D (p=.06), which translates to an 11% relative decrease.
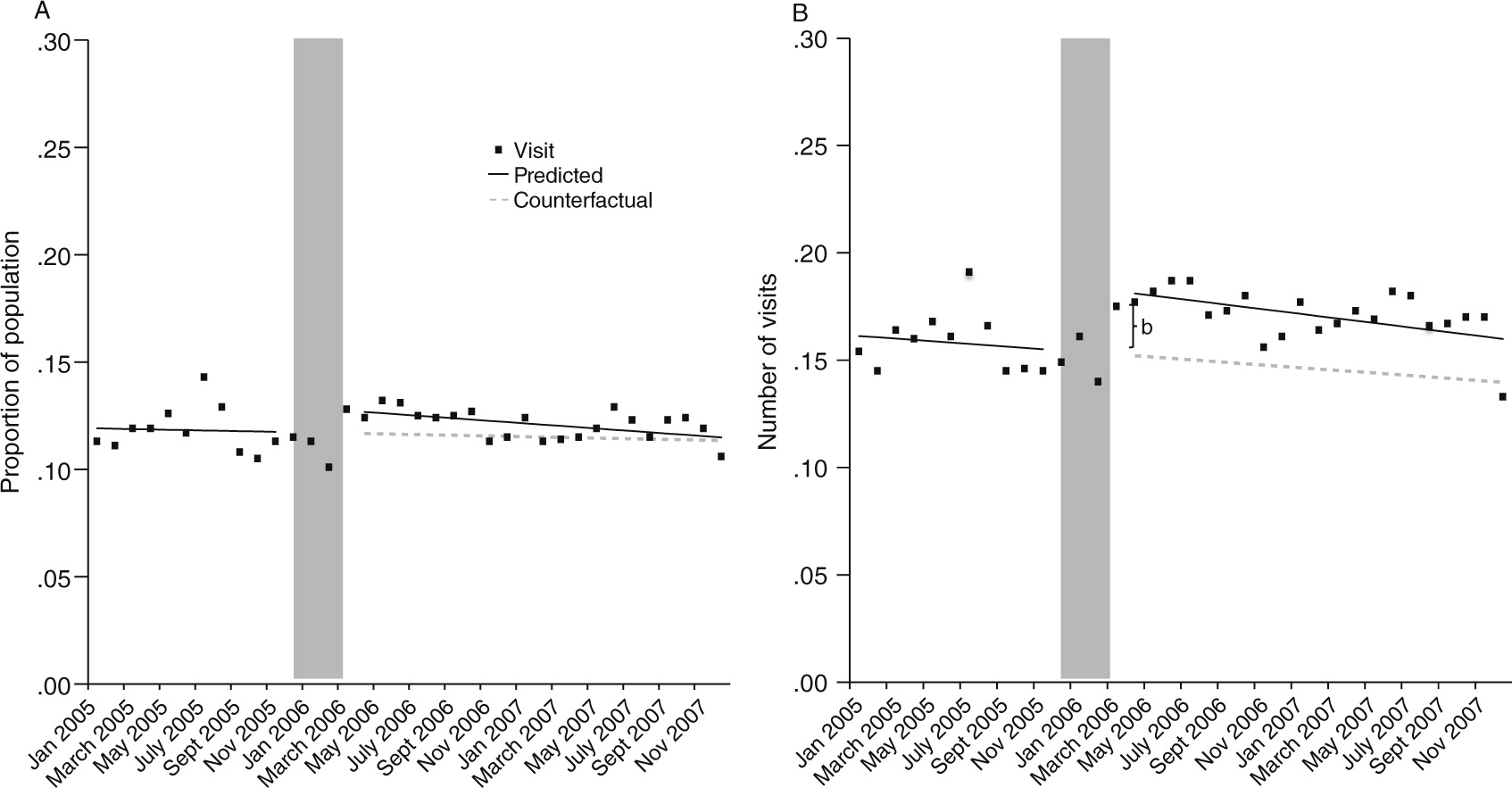
The transition to Part D coverage did not alter the probability of having any emergency department visit in the month (
Figure 2A and
Table 2). However, there was a 19% relative increase (.03/.16 visits) in the mean number of monthly emergency department visits for any cause immediately after the transition to Part D coverage (
Figure 2B and
Table 2). The estimated difference between predicted and expected number of emergency department visits attributable to Part D as of April 2007 had very wide confidence intervals and was not statistically significant (
Table 2).
We also detected no change in the probability of having any emergency department visit related to mental health or substance use (
Table 2). We did, however, observe a 14% relative increase (.01/.07 visits) in the mean number of visits per month related to a mental health or substance use disorder (
Table 2).
Discussion
We evaluated the effects of the transition from Medicaid to Part D prescription drug coverage on receipt of recommended pharmacotherapy and emergency department visits among dually enrolled beneficiaries with a diagnosis of bipolar I disorder. There were two notable findings. The coverage transition did not adversely affect receipt of guideline-concordant pharmacotherapy; rather, it was associated with very modest improvements. Second, although there was no significant change in the mean monthly rate of beneficiaries with any emergency department visit throughout the study period, there was an increase in the mean number of emergency department visits per month immediately after the transition to Part D coverage.
The transition to Part D coverage flattened a previously declining trend in receipt of recommended antimanic medication. The declining trend in antimanic medication use before Part D implementation coincided with a period in which Medicaid programs increasingly used prior authorization to manage its use (
10). The introduction of these state-level policies has been associated with relatively lower use of antimanic medications (
10). The observed decline in receipt of antidepressant monotherapy after the coverage transition might be explained by greater receipt of antimanic medications as a result of relaxation of state policies targeting their use. Receipt of this contraindicated pharmacotherapy may partially result from limited access to recommended antimanic medication rather than to patient or provider preference.
Our results for emergency department use indicate that visits increased only among a subset of beneficiaries who had at least one visit in the month. In addition, this increase was concentrated in the immediate posttransition period and was followed by a gradual decline, suggesting that the transition is an important period of vulnerability. The estimated increase would equate to 43 additional emergency visits per month from a baseline of 229 emergency visits per month for this sample of 1,431 beneficiaries until the immediate posttransition increase began to dissipate. This rise in visits may signal medication treatment discontinuity or drug switching associated with the transition period. Although we did not observe a reduction in receipt of evidence-based pharmacotherapy coincident with the transition, our outcome measures were not sensitive to all types of treatment discontinuities, including switching between medications and within-month treatment gaps (
8).
Medicaid beneficiaries continue to transition to Medicare and Part D prescription drug coverage each day. As soon as they become eligible for Medicare, beneficiaries are randomly assigned to a Part D plan. The experience of today’s “transitioners” may differ from that of the initial cohort of our study, given subsequent program changes. The prevalence of utilization management requirements for psychiatric medications has increased among Medicare private drug plans (
14). However, in 2008 the Centers for Medicare and Medicaid Services implemented a grandfathering provision that applies to the protected drug classes (antipsychotics, anticonvulsants, and antidepressants). Plans may not implement prior-authorization or step therapy requirements intended to steer beneficiaries to a new medication if they were already taking a drug within that class at the time of enrollment (
5). Today, continuity and adequacy of pharmacotherapy for beneficiaries with bipolar I disorder who are transitioning to Part D coverage may be determined in large part by individuals’ treatment history while under Medicaid drug coverage.
This study aimed to make inferences to the nonelderly adult dual-beneficiary population with bipolar I disorder, who are individuals with various levels of severity, duration, and phase of illness. We required continuous enrollment, so our results may not generalize to less severely ill beneficiaries who exited the program. This research design allowed us to estimate the national average effect of the transition from Medicaid to Part D on study outcomes. We could not identify the mechanisms within this transition that accounted for the observed effects. Medicaid programs and Medicare prescription drug plans (PDPs) varied in their prescription drug coverage and management. Thus our results may mask differences across states (given variation in Medicaid programs) and within states (given variation in PDPs). Finally, our measures of guideline concordance may conceal questionable practices concerning quality of care (such as inappropriate polypharmacy) that are not discernible from claims data alone.
Conclusions
There was significant concern before the implementation of Part D regarding the potential effects of changing the drug coverage for dual beneficiaries with serious mental illness (
4). We found that the likelihood of receiving evidence-based pharmacotherapy for bipolar I disorder did not decline; however, the immediate rise in emergency department visits among a subgroup of beneficiaries after the transition requires further investigation.
Acknowledgments and disclosures
The authors gratefully acknowledge the following grant support: 1K01MH092338 from the National Institute of Mental Health; 1R01-HS018577-01 from the U.S. Agency for Healthcare Research and Quality (AHRQ), awarded to the Department of Population Medicine at Harvard Medical School and Harvard Pilgrim Health Care Institute where this study originated; a Robert Wood Johnson Foundation Investigator Award in Health Policy Research, awarded to Dr. Huskamp; 5R01AG032249 from the National Institute on Aging; and R01 MH084905 from AHRQ.
The authors report no competing interests.