In Alzheimer’s disease (AD), decreases in regional cerebral blood flow (rCBF) and metabolism are reported to precede and to be more severe than decreases in cognitive function and brain volume.
1,2 Several studies have examined longitudinal changes in regional cerebral perfusion
3–6 or glucose metabolism
1,2,7–9 in patients with AD by using single photon emission computed tomography (SPECT) or positron emission tomography (PET). Most of these studies adopted a conventional region of interest technique. As region of interest analyses focus on regions selected according to an a priori hypothesis, they may overlook substantial changes in the “uninteresting” areas.
In this study, changes in cerebral glucose metabolism that occurred in patients with AD over a 1-year period by using [
18F]-2-fluoro-deoxy-
d-glucose (FDG) PET were examined. A voxel-by-voxel analysis, which avoids the risk of overlooking regions that may be important was also used.
10 As the pattern and rate of disease progression in AD may depend on the disease stage,
11,12 only patients in the early stage of AD were included.
METHODS
This study was conducted at Hyogo Institute for Aging Brain and Cognitive Disorders, a research-oriented hospital for dementia. All procedures followed the Clinical Study Guidelines and the PET Drug Usage Manual, Ethical Committee, Hyogo Institute for Aging Brain and Cognitive Disorders, 1993, and were approved by the Institutional Review Board. Written, informed consent was obtained for all patients and their caregivers according to the Declaration of Human Rights, Helsinki, 1975.
One hundred thirteen patients with AD who fulfilled the inclusion criteria were examined by using PET between March 1994 and February 1998. All patients were examined by both neurologists and psychiatrists and were given routine laboratory tests. Electroencephalograms, magnetic resonance imaging (MRI) of the brain, and MR angiography of the head and neck were obtained for all patients. The inclusion criteria for PET were 1) the criteria of the National Institute of Neurological Disease and Stroke/Alzheimer’s Disease and Related Disorders Association for probable AD
13 and 2) minimal to mild functional severity (grades 0.5 and 1) on the Clinical Dementia Rating Scale.
14 Excluded were patients 1) with medical illnesses possibly causing cognitive impairment, including thyroid diseases, vitamin deficiencies, and malignant diseases; 2) patients with developmental abnormalities, substance abuse, or significant neurological antecedents such as brain traumas, brain tumors, epilepsies, and inflammatory diseases; 3) patients with history of previous mental illnesses such as depression before onset of dementia; 4) patients with evidence of focal brain lesions on MRI, including lacunar infarcts and hematoma; 5) patients with left- or both-handedness; and 6) patients whose informed consent was not obtained. Patients with white matter or subcortical hyperintense lesions on T
2-weighted images, which were not shown on T
1-weighted images, were not excluded.
Among those patients, 29 had a follow-up PET examination after a 1-year interval (mean=395 days, SD=32) as part of an annual follow-up program.
15 The subjects consisted of 17 women and 12 men, and the mean age at baseline examination was 66.1 years (SD=8.5), while the mean educational attainment was 9.8 years (SD=2.8). The mean duration of symptoms, which was determined through an interview with the primary caregiver and defined as the time between the first appearance of symptoms of sufficient severity to interfere with social or occupational functioning and admission,
16 was 32.1 months (SD=22.9). The apolipoprotein E genotyping was ϵ3/ϵ3 in 11 patients, ϵ3/ϵ4 in 12 patients, and ϵ4/ϵ4 in six patients. The mean baseline and follow-up scores on the Mini-Mental State Examination
17 were 22.5 (SD=2.7) and 21.0 (SD=4.2) (
t=2.99, df=28,
p=0.0058, two-tailed, paired t test), and mean scores on the Alzheimer’s Disease Assessment Scale Cognitive Subscale
18 were 15.0 (SD=4.8) and 18.0 (SD=8.4) (
t=–2.23, df=28,
p=0.031, two-tailed, paired t test).
This study started before the approval of donepezil hydrochloride in Japan (donepezil hydrochloride is only one drug licensed in Japan for the treatment of AD and launched in November 1999), and none of the patients received any anti-AD drugs during the study period. The PET data from one part of these 29 patients had been used in our previous studies.
19–25The detailed PET procedure is described elsewhere.
26 In brief, fluorodeoxyglucose-positron emission tomography (FDG PET) images were obtained using a Headtome IV scanner (Shimadzu Corp., Kyoto, Japan). All subjects had fasted for at least 4 hours before PET scanning. Subjects were studied under resting conditions with eyes closed and ears unplugged. After a transmission scan, a 12-minute emission scan was started 60 min after an intravenous injection of 185–370 MBq of FDG. We used NEUROSTAT (University of Michigan, Ann Arbor, MI, USA) for anatomical standardization of PET images, as brain atrophy is sometimes seen in AD patients. In our previous study,
27 we noted that NEUROSTAT was more suitable than Statistical Parametric Mapping 99 (SPM 99, The Wellcome Department of Neurology, London, UK) for anatomical standardization of atrophied brains. The image sets were transformed to a standard stereotactic space on a Power Mac G4 computer (Apple, Cupertino, Calif.) using the part of the program NEUROSTAT that generates standardized three-dimensional stereotactic surface projections data sets for individual subjects. Then, regional cerebral metabolic images were compared by using SPM 99. The PET images were smoothed using an isotropic Gaussian filter of 12 mm diameter to compensate for intersubject gyral variability and to reduce high frequency noise. Finally, after individual global counts were normalized by the glucose metabolism of the pons,
28 within-group comparisons between initial and follow-up examinations were performed on a voxel-by-voxel basis on all voxels common to each subject,
10 The thresholds were set at
p=0.001. Correction for multiple comparisons was not performed.
DISCUSSION
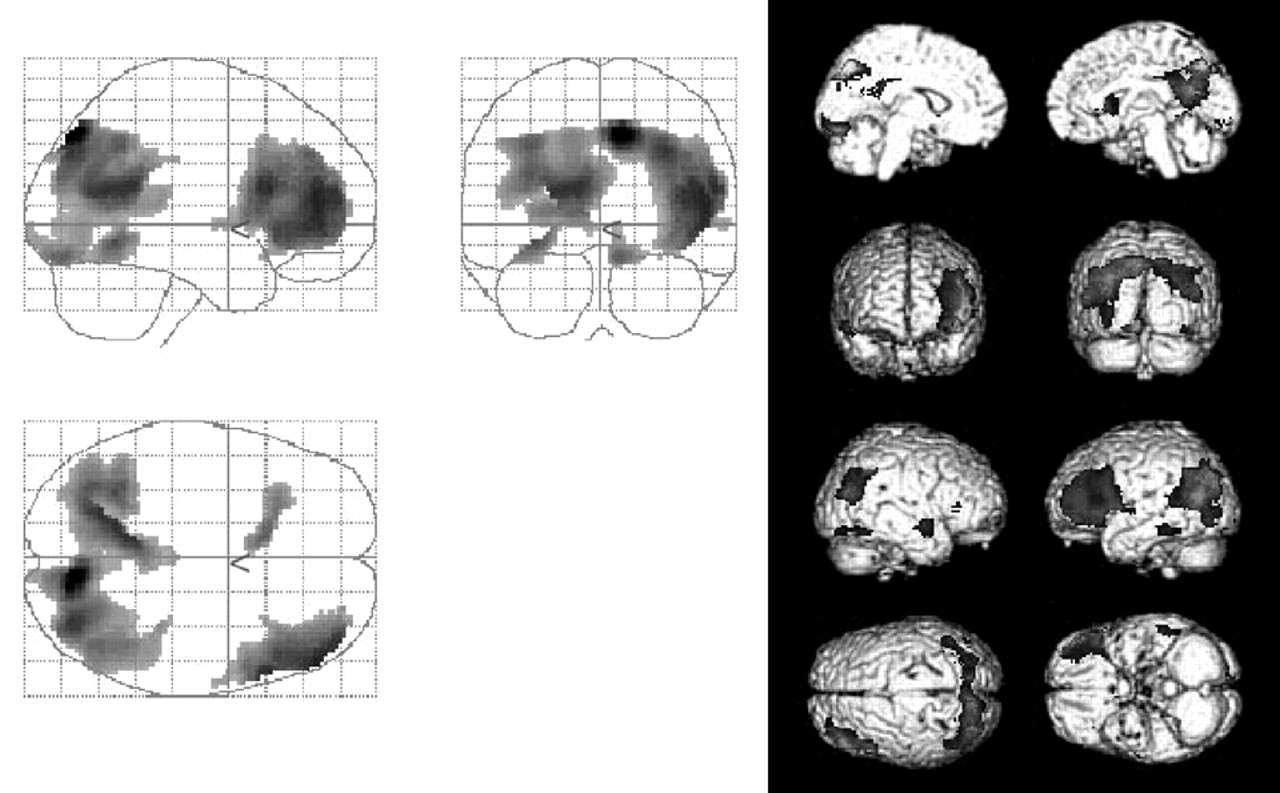
This study clearly demonstrates a significant involvement in the parietal association cortices and temporal and frontal gyri. In addition to these regions, we found significant involvement in the bilateral precunei and posterior cingulate gyri. The former findings are consistent with the results of previous studies,
1,2,4–6 whereas the latter findings were not previously detected in longitudinal functional neuroimaging studies. However, a selective decrease in rCBF and/or glucose metabolism in these areas has been reported in the very early stage of AD.
6,29–31 Our findings are consistent with the concept that the brain regions that show functional changes also show further deficits,
2 at least over a 1-year period during the early stage.
The medial temporal structures including the hippocampal formation, entorhinal cortex, and amygdala are initially affected in AD.
32 In the early stage of AD, a significant atrophy of these structures has been revealed by MRI morphometry,
33 and a significant reduction of cerebral blood flow has been demonstrated.
6 However, we failed to detect a significant change of glucose metabolism in the medial temporal areas. Similarly, recent PET studies failed to find any significant decreases in glucose metabolism in the medial temporal regions, even when glucose hypometabolism was noticeable in the parietal association cortices.
34,35 Taking these findings into consideration, metabolic changes in the medial temporal structures may be mild or difficult to detect in the early stage of AD. Thus, the metabolic changes in these regions are not appropriate for monitoring the progression of AD or making an early diagnosis of the disease.
The generalizability of this study is limited by the hospital-based, small-scaled study design. The selection of subjects was unintentional but possibly biased. Another methodological limitation was the use of SPM for voxel-by-voxel analysis. Statistical parametric mapping was developed originally as an analytical tool for PET activation studies of healthy volunteers and was not intended to use in studies of patients with brain disease. It has been reported that potential artifacts can be introduced when SPM is applied to atrophic brains.
27 Nevertheless, SPM has been commonly used for voxel-by-voxel analysis in studies on AD and generated a number of significant findings. Furthermore, in order to reduce possible artifacts, we made use of NEUROSTAT, an alternate software for anatomical normalization and registration, of which performance was more accurate than SPM when applied to atrophic brain.
27As mentioned above, decreases in rCBF and glucose metabolism are reported to precede and to be more pronounced than decreases in cognitive function and brain volume.
1,2 Our findings suggest that evaluation of glucose metabolic changes in the parietal, temporal, and frontal cortices, precunei, and posterior cingulate gyri (but not in the medial temporal structures) can be useful not only for the early diagnosis of AD but also for monitoring the progression of AD in its early stage. Functional brain imaging can also be a valid and useful surrogate outcome measure for anti-Alzheimer’s disease drug trials, as it would be more sensitive than cognitive measures or morphological brain imaging.