Multiple sclerosis (MS) is the most common nontraumatic neurological illness among young adults. The disease is characterized by a progressive course and affects multiple areas of the central nervous system (CNS). Following the appearance of neurological symptoms, MS is diagnosed by means of the patient’s history, cerebral spinal fluid (CSF) analysis, magnetic resonance imaging (MRI), and evoked potential recordings.
1–3Even in early stages of MS, patients and healthy comparison subjects may differ in cognitive test performance. Multiple sclerosis patients may demonstrate memory deficits in particular.
4–11Before magnetic resonance spectroscopy was available as a clinical tool, conventional MRI-data (i.e., plaque volume, plaque localization, and brain atrophy) were compared with neuropsychological test scores to correlate structural damage with cognitive function.
12–14Conventional magnetic resonance (MR)-techniques have significant limitations in the diagnosis of MS due to poor specificity for hyperintense changes, particularly in gray matter, and for the detection of changes in otherwise normal appearing white matter. These are two of the main reasons why the correlation between T2 lesion volume and neurological disabilities is only modest in patients with multiple sclerosis.
In vivo proton magnetic resonance spectroscopy (
1H-MRS) was developed to assess biochemical changes that occur in many diseases of the brain. The use of
1H-MRS to measure
N-acetylaspartate (NAA) offers insight into neuronal processes, revealing a high concentration of NAA disturbances among patients, predominantly in neuronal structures.
15–16A decrease of NAA concentration in brain lesions of MS patients and in normal appearing white matter (NAWM) and its correlation with clinical disabilities has been described in the literature.
17–23Discrepancies concerning frontal activity during memory processes between functional imaging and neuropsychological data may indicate a limitation or insensitivity in behavioral measures. Fletcher and Henson
24 suggest that functional imaging data contain important information about the way subjects perform memory tasks.
In the present study, we hypothesized that lower levels of memory in early stages of MS are accompanied by a reduction of NAA in cortical gray matter, which is relevant to cognitive function. Supporting a role of anterior cingulate cortex in a memory network, Fazio et al.
25 found bilateral metabolic reductions in the hippocampal formation, thalamus, cingulate gyri, and frontal basal cortex in a positron emission tomography (PET) study. We determined the NAA/Creatine (Cr) ratio in frontal deep white matter and in the cortex of the frontal cingulate gyrus (Brodmann areas 24/32) to be part of the cognition division of the anterior cingulate cortex (including caudal areas 24 and 32, the cingulate motor areas in the cingulate sulcus, and nociceptive cortex). Brodmann areas 24 and 32 are involved in cortical circuits responsible for brain function that requires cognitive processing. Area 24 may involve tasks that require short-term memory but that do not require movement. Tasks requiring target assessment and attention to linguistic and other features of the sensory environment may engage area 32.
26,27METHODS
Patients
Our sample included 21 patients between the ages of 18 and 43 years (mean 33.5; SD=7.5) who were diagnosed according to the criteria of Poser et al.
28 and were suffering from definite MS with a relapsing-remitting course for less than 3 years. Only patients without frontal atrophy were included, and the same effect of CSF was deduced in all subjects.
Patients were compared to 21 healthy volunteers who were matched according to right handedness, age, years of education, and sex. Volunteers were between 20 and 45 years of age (mean 31.8; SD=7.4). All subjects signed a written consent form.
Measures
Disability was assessed by using the Expanded Disability Status Scale (EDSS).
29Expanded Disability Status Scale scores of the MS patients did not exceed 2.5 (Median=1.5; Range=0–2.5). No subject had a history of psychiatric disorder.
All subjects were interviewed and tested neuropsychologically using the Wechsler Memory Scale (WMS),
30 a standard clinical memory assessment procedure that consists of seven subtests summarizing in a total score. Subjects were also tested using the Multiple Sclerosis Functional Composite (MSFC) scale,
31 an outcome measure for MS clinical trials that assesses the dimensions of ambulation/leg function, arm/hand function, and cognition.
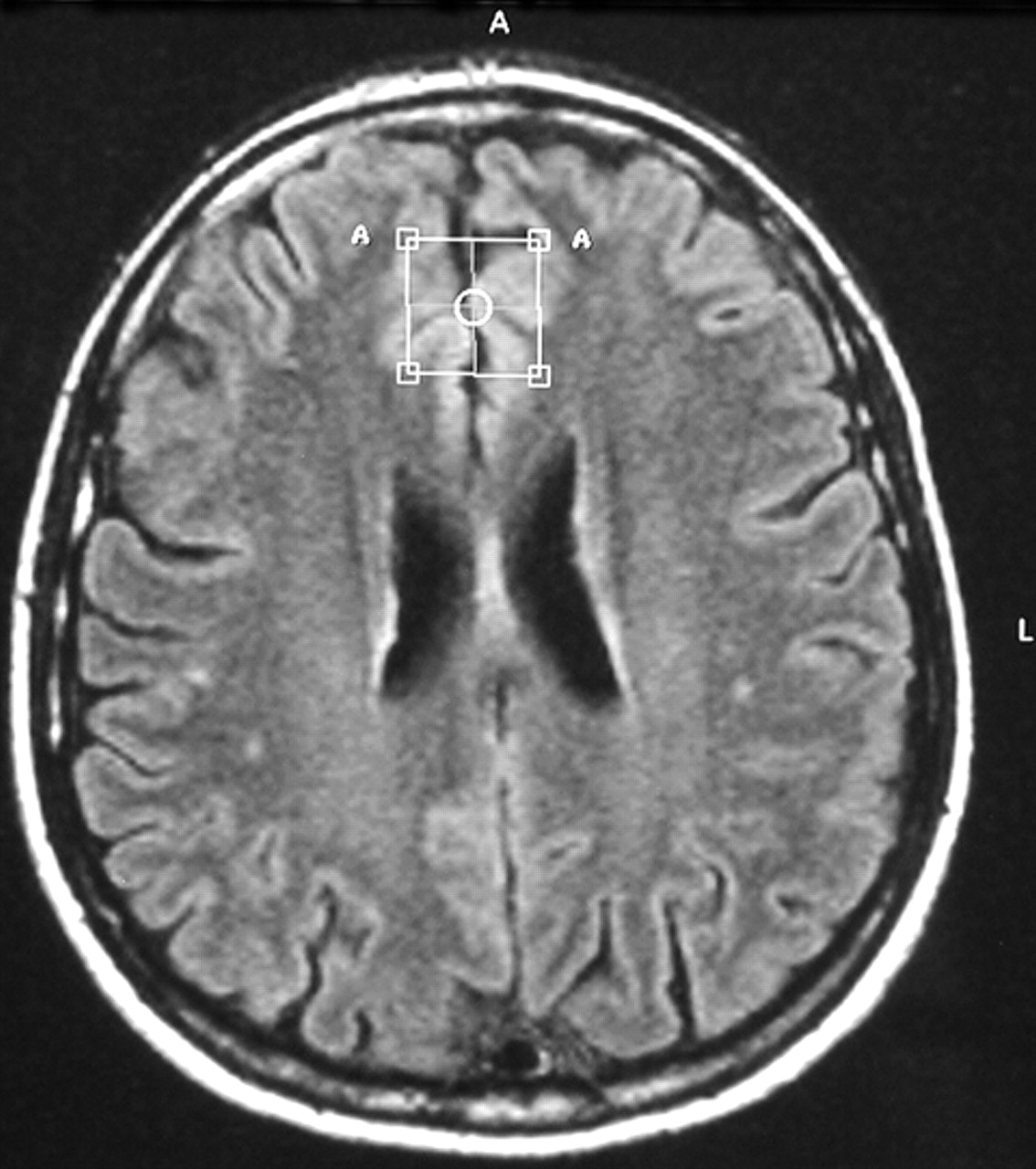
Conventional proton MRI was performed with axial T2 weighted fluid attenuated inversion recovery (FLAIR) images (22 slices, slice-thickness 5 mm, matrix 256×256, TR = 4452 msec; TE = 100 msec) and coronal T1 weighted scans (22 slices, slice-thickness 6 mm, matrix 256×256, TR = 560 msec; TE = 14 msec). In vivo proton magnetic resonance spectroscopy examinations of the brain were obtained in a single session using the 1.5 T Philips Gyroscan (Philips Medical Systems, Best, the Netherlands). Axial and coronal images were used to position the volume of interest (VOI) (size 2×2×2 cm
3) in the left frontal deep white matter and gray matter of the frontal cingulate gyrus (Brodmann areas 24/32) of both hemispheres. Voxel positioning was done visually according to a template image taken from the atlas of Talairach and Tournoux.
32 White matter voxel center coordinates were x=−34, y=10, N=27 (origin: anterior commissure). The voxel center of the gyrus cinguli was positioned to the coordinates x=0, y=27, N=27, and predominantly gray matter structures were within the VOI (
Figure 1).
We used a PRESS technique (TR = 2000 msec; TE = 136 msec) with automatic shimming, water suppression by frequency selective adiabatic pulse, time domain filtering by Gaussian, and exponential multiply. Spectra analyses were conducted by measuring peak NAA and Cr. Results were expressed as NAA/Cr ratio, assuming Cr as a stable metabolite.
33Statistics
Comparison between groups was computed using the Chi-Square Test for nominal data and the Mann-Whitney U-Test (two-tailed) for ordinal scaled data. Linear multiple regression analysis (stepwise method) was computed to examine the significance of MRS data an independent variable of memory outcome (SPSS 10.0).
RESULTS
Conventional MRI showed small plaque volumes in all patients. Single plaques in deep white matter of the frontal lobe were found in 33% of the patients. No plaques were found in frontal gray matter or subcortical white matter.
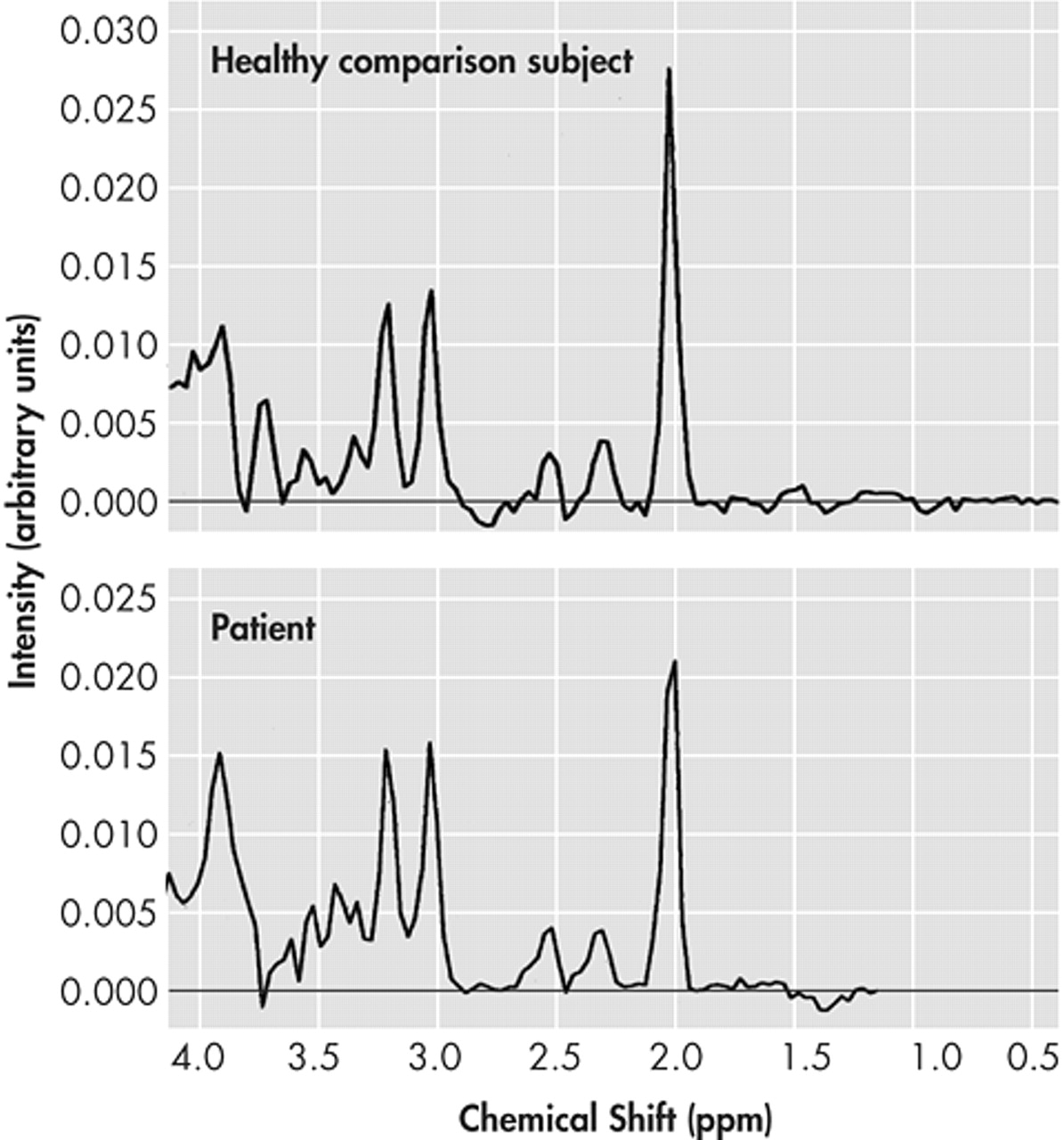
The NAA/Cr ratio of patients in the frontal cingulate gyrus showed a significant reduction (p=0.004) when matched against healthy comparison subjects (
Figure 2).
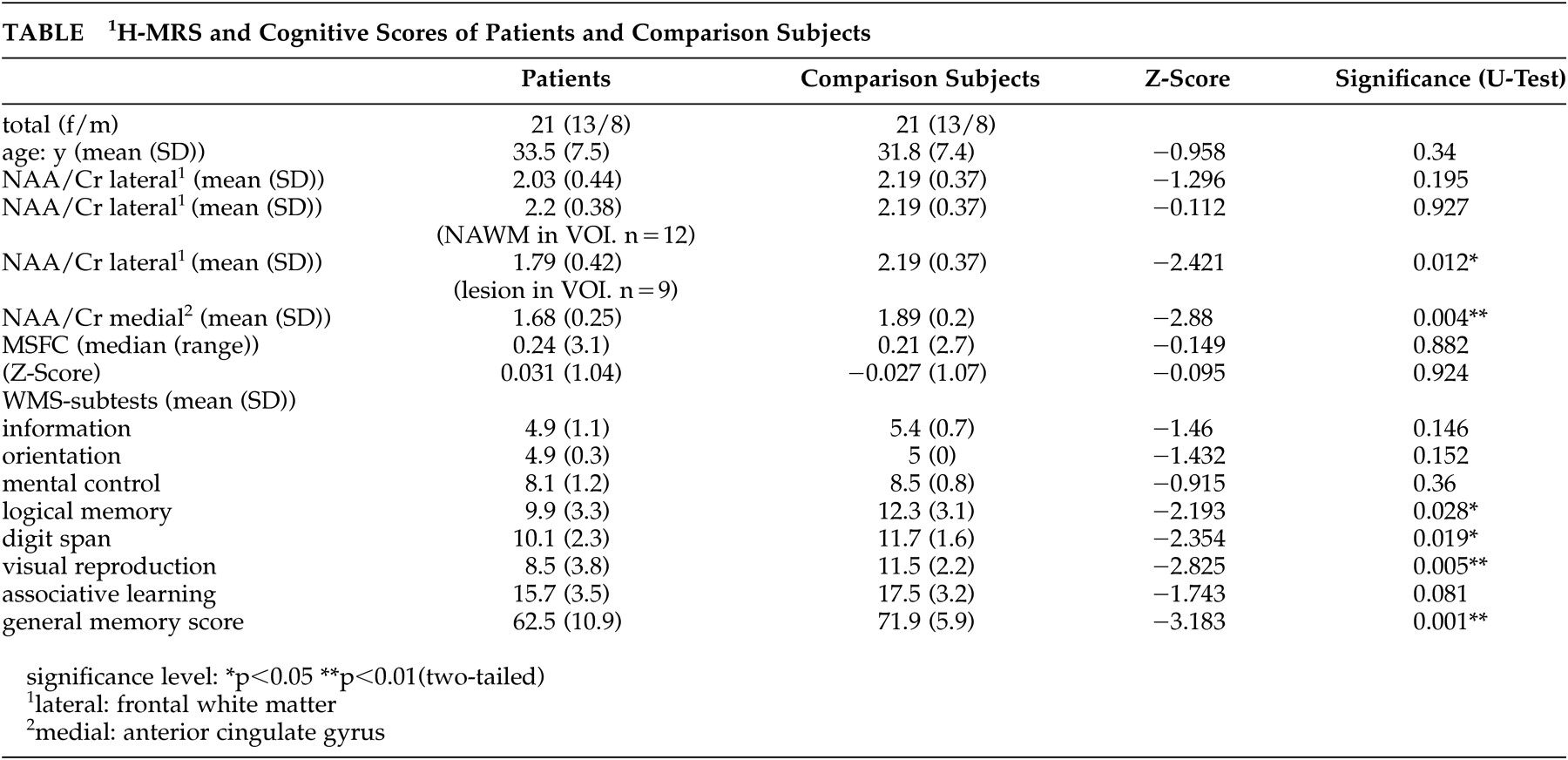
No significant reduction of the NAA/Cr ratio was found in the VOI positioned in the frontal white matter when the whole patient group was compared with healthy subjects (p= 0.195). Dividing patients according to white matter lesions in VOI, the NAA/Cr ratio of the group without lesions (N=12) did not significantly differ from that of healthy subjects (p=0.927). As we anticipated, the group with lesions in white matter VOI (N=9) significantly differed from healthy comparison subjects (p=0.012), as expected.
The multiple sclerosis functional composite scores of patients and healthy subjects did not significantly differ. Multiple sclerosis patients scored significantly lower than healthy comparison subjects in WMS total score (p=0.001). This was also the case for the WMS subtests in logical memory (p=0.028), digit span (p=0.019), and visual reproduction (p=0.005) (
Table).
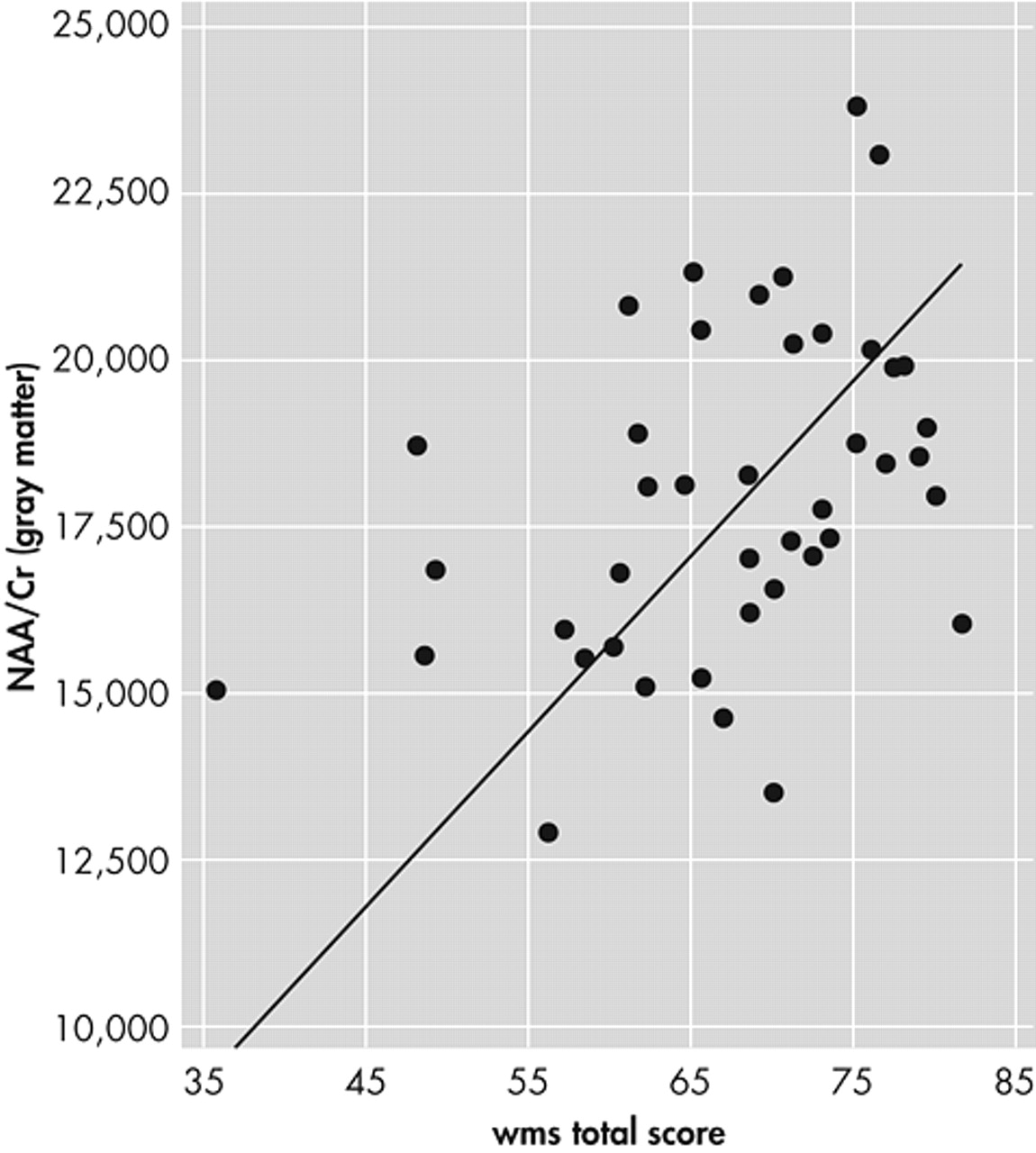
Using NAA/Cr ratios of gray and white matter as independent variables for WMS-scores, linear multiple analysis regression (stepwise) for all subjects showed gray matter NAA/Cr ratio of the frontal cingulate gyrus to be significantly associated with the WMS total score (N=8.105, df=1; p=0.007) (
Figure 3); WMS associative learning (N=7.138, df=1; p=0.015); and WMS logical memory (N=6.238, df=1; p=0.016). White matter NAA/Cr ratio was not a significant independent variable in regression analysis for any cognitive test score.
DISCUSSION
Combining
1H-MRS metabolite measurements with cognitive capacities provides new insight into understanding neuropsychological disturbances caused by chronic diseases of the brain such as MS.
34–37In vivo proton magnetic resonance spectroscopy studies of white matter in MS patients
38–45 have shown significant correlations between the reduction of NAA and clinical disabilities.
17–23 Other studies have found significant associations between cognitive function and the reduction of NAA in white matter.
46–48 Recent studies have reported the involvement of gray matter structures in MS patients,
49–52 but these studies do not address the correlation between gray matter and cognitive functions.
In our study using in vivo proton magnetic resonance spectroscopy, VOIs were located in the left frontal deep white matter and in the frontal cingulate gyrus (Brodmann areas 24/32), which is an important cortical area for cognitive functions such as attention focusing, short-term memory, and working memory.
27,53,54 To minimize the partial volume effect caused by CSF, the voxel was positioned as closely as possible to these Brodmann areas and subjects with atrophy were excluded.
We detected significant reduction of the NAA/Cr ratio in frontal white matter when MS plaques were localized within the VOI. The NAA/Cr ratio in NAWM was not reduced in our patient group, which was in accordance with the low clinical disability.
Also indicating a distinct disturbance of gray matter in MS patients, a significant reduction of NAA/Cr ratio in the frontal cingulate gyrus was found among this patient group when compared to healthy subjects.
Considering the well preserved clinical integrity of the patients in this study, the MS-specific MSFC did not show any significant difference between patients and healthy comparison subjects, which is what we had expected.
We found significant differences in memory abilities, as measured by the WMS general memory score and its subtests: logical memory, digit span, and visual reproduction. These findings are in agreement with the results of a metaanalysis of 36 studies investigating memory deficits in MS-patients.
10 This analysis suggests a global memory disorder containing indices for short-term memory, working memory (in particular the simultaneous retention and internalization of information), and long-term memory among MS patients, which differed significantly from that of comparison subjects.
In our sample (patients and healthy comparison subjects), no significant relation between NAA/Cr ratio in white matter and memory scores was found. The NAA/Cr ratio in gray matter of the frontal cingulate gyrus, however, was a significant independent variable in regression analysis, predicting overall memory score and logical and verbal associative memory. These findings may stimulate further debate about the general relation between memory function and NAA-concentration in the cortices.
We conclude that metabolic changes of the cerebral cortex, such as reduction of NAA/Cr ratio in gray matter, may be a sensitive indicator of cognitive function. Therefore, further research should focus on relating additional gray matter metabolic brain mapping with cognitive functions.
ACKNOWLEDGMENTS
This study was presented at the European Federation of Neurological Societies (ENS) Congess, Vienna, October 2002.