T here is increasing interest in conceptualizing schizophrenia-spectrum illnesses beyond the traditional diagnostic elements of positive and negative symptoms, moving toward a more comprehensive understanding that includes associated features such as cognitive deficits and affective symptoms. While these symptoms may not be specific to schizophrenia, their strong association with chronicity of illness and aspects of impaired functional outcomes justify the interest for their characterization and treatment. A review of studies examining the frequency of depressive episodes in schizophrenia found prevalence rates ranging from 7% to 75%.
1 The level of chronicity of the illness is an important factor contributing to the variance in these estimates.
2 Depressed mood was reported to be commonly associated with first-episode schizophrenia and to occur mostly in association with acute psychotic episodes.
3 However, Siris et al.
4 reported the presence of depression in one-third of schizophrenia patients several months after the remission of a psychotic episode, an event that has been more recently termed “post-psychotic depression” in schizophrenia.
5 Furthermore, longitudinal studies examining the course of schizophrenia have found depressive symptoms to be prevalent during all stages of this disorder.
6,
7 Another factor contributing to differences in the reported frequencies of depressive symptoms in schizophrenia is the variety of instruments used to assess the presence and severity of these symptoms.
2 Widely-used depression rating scales are often employed for the assessment of depression in schizophrenia.
8 However, the use of standard depression rating scales in this disorder has been criticized as inappropriate because these rating instruments have been designed and validated only for nonpsychotic populations with a diagnosed depressive illness.
9 The Calgary Depression Scale was developed by Addington et al.
10 specifically for the assessment of depressive symptoms in schizophrenia. Collins et al.
11 demonstrated the methodological superiority of the Calgary Depression Scale in the assessment of depressive symptoms in this illness over scales developed for nonschizophrenic populations such as the Hamilton Depression Rating Scale (HAM-D)
12 and the Present State Examination.
13 In spite of this, when used to assess schizophrenia outpatient populations, scores from the Beck Depression Inventory (BDI),
14 a depression rating scale originally developed to be used in nonpsychotic patients, have been shown to be significantly correlated with scores from the Calgary Depression Scale.
15 Also, in contrast to the HAM-D and the Calgary Depression Scale, the BDI is a patient self-rated scale. Thus, by focusing on self-reported features of depression, this scale may constitute a useful tool for the measurement of changes in subjective mood during treatment. The use of the BDI, with established validity across neuropsychiatric conditions, has the added advantage of allowing comparisons between schizophrenia and nonschizophrenia populations.
There is emerging evidence that depressive symptoms, regardless of how they are assessed, are associated with impairment in everyday functions,
16 poorer quality of life,
17 and greater need for medication and hospitalization
18,
19 in patients with schizophrenia. Moreover, Siris et al.
20 have reported that depressive symptoms increase mortality rates in these patients by contributing to their alarmingly high rates of suicide. However, the relationship of depressive symptoms and functional outcomes in schizophrenia has not been fully elucidated. Bowie et al.
21 recently demonstrated that the severity of self-reported depression in schizophrenia did not have an impact on the patients’ neuropsychological performance or scores on performance-based measures of functional capacity. However, depressive as well as negative symptoms affected the likelihood of successful deployment of functional skills during everyday life tasks (i.e., real-world functional performance).
The functional significance of depression in schizophrenia is obscured by its conceptual and operational overlap with the negative symptoms found in this disorder.
22 Feelings of guilt and suicidal ideation may be suggestive of depression. However, features such as psychomotor retardation, impaired ability to concentrate, and decreased interest, pleasure, energy, or motivation could be part of negative as well as depressive symptomatology.
1 While numerous authors have reported a strong association between depressive and negative symptoms of schizophrenia,
23 –
25 others have found significantly higher correlations between depression and positive symptoms compared to negative ones.
26,
27 Lysaker et al.
27 further hypothesized that the lack of correlational relationship between depression and negative symptoms is to be expected since negative symptoms, in contrast to positive ones, represent a deficit state with significantly reduced capacity for internal experience. Further evidence for the discriminant validity of negative symptoms and depression in schizophrenia was provided by reports of greater improvement in depressive, as compared to negative symptoms after the use of antipsychotic medication.
28Studies examining the overlap between depression and classical schizophrenia symptoms with total scores have reported contradictory findings. One possible explanation for inconsistent findings is the fact that depression may be a multidimensional entity, with potentially different dimensions in schizophrenia and other psychiatric disorders. There is evidence from other neuropsychiatric conditions such as multiple sclerosis,
29 chronic pain,
30 and Parkinson’s disease
31 that more than one symptom factor emerges from the BDI. It is unknown whether the BDI is multifactorial in schizophrenia or if these different components have differential relationships with clinical symptoms or outcomes.
The purpose of this report is to examine the independent and overlapping features of negative and depressive symptoms in a large sample of outpatients with schizophrenia. The dimensional structure of these symptom dimensions was examined with multivariate statistical techniques while correlational analyses related the results of these analyses to each other across the symptom domains of depression and negative symptomatology. Correlates of the total score and domains with ability and performance areas were examined with bivariate correlations. It was our hypothesis that depression in schizophrenia would be multifactorial in nature and that different components would have differential correlations with other symptoms of the illness.
METHODS
Participants
Participants were older schizophrenia outpatients enrolled in a longitudinal study of the course of cognitive and functional status. Exclusion criteria consisted of a primary DSM-IV axis I diagnosis other than schizophrenia or schizoaffective disorder, Mini-Mental Status Examination (MMSE)
32 score below 18, Wide-Range Achievement Test, 3rd ed. (WRAT-3)
33 reading grade-equivalent of grade 6 or less, or any medical illnesses that might interfere with the assessment of cognitive functioning. All subjects met diagnostic criteria for schizophrenia or schizoaffective disorder. The Comprehensive Assessment of Symptoms and History (CASH)
34 was completed by a trained research assistant (HA) and diagnosis was confirmed by a senior clinician (EC). In addition, patients’ data were used in these analyses only if they were receiving case management services and actively involved in psychiatric rehabilitation services. Study participants were also required to have evidence of continued illness at the time of recruitment, as evidenced by meeting at least one of three criteria: an inpatient admission for psychosis in the past 2 years; an emergency room visit for psychosis in the past 2 years; or a score on the Positive and Negative Syndrome Scale (PANSS),
35 positive symptoms items delusions, hallucinations, or conceptual disorganization, of 4 (moderate) or more at the time of baseline assessment. Outpatient status was defined as living outside of any institutional setting, including a nursing home. Recruitment was conducted at clinics at Veterans Affairs Hospitals, New York State Psychiatric Hospital, or the Mount Sinai School of Medicine.
All patients were receiving treatment with antipsychotic medications at the time of assessment. After the testing procedures were fully explained, all participants signed a written informed consent form approved by the institutional review board at each research site.
Measures
All participants completed the test battery in a fixed order. Screening measures were of global cognition with the MMSE and estimated premorbid functioning with the WRAT-3 Recognition Reading subtest. These measures were followed by functional skills assessment, a cognitive test battery, and a symptom interview. All interviewers received extensive training in performing all assessments, and every 3 months their performance was evaluated through re-rating of training tapes, dual-ratings of the functional status measures with the first author, and quality assurance assessments of all testing. These raters were trained to adequate reliability on symptom ratings with two full days of training, four standardized videotapes, and in-person interviews that yielded intraclass correlation coefficients from 0.86 to 0.92. Case managers rated the Specific Level of Function Scale (SLOF),
36 which provides an assessment of objective real world performance in domains that include work skills, participation in community activities, socially acceptable behavior, and interpersonal performance.
Symptom Assessment
Severity of schizophrenia symptoms was assessed using the Positive and Negative Syndrome Scale. This is a 30-item scale with seven items measuring positive symptoms, seven items measuring negative symptoms, and 16 items measuring general aspects of psychopathology, and is completed after a structured interview. We obtained self-reports of depression from patients using the Beck Depression Inventory-II.
37 The original BDI is a 21-item inventory to measure the severity of depressive symptoms. The items are based on Beck’s observations of the symptoms and attitudes of depressed persons seen in the context of therapy. Consequently, Beck et al.
37 created a revised version of the BDI. The main changes made to develop the BDI-II primarily reflected increased compatibility with the DSM-IV. This scale is self-administered, requires only about 5–10 minutes to complete, and can be used with persons ages 13 years and up. Each item in the BDI is rated on a scale of 0–3, and scores from all items are tallied to obtain a total possible score, ranging from 0 and 63, with higher scores reflecting greater severity of depressive symptomatology. Scores between 0 and 9 are interpreted as “minimal” depression, scores between 10 and 15 as “mild” depression, scores of 16–20 as “mild/moderate” depression, scores of 20–29 as “moderate/severe,” and scores of 30 and greater as “severe” depression.
38Skills Assessment
Cognitive and functional skills were assessed with performance-based measures in the laboratory. Patients completed a comprehensive assessment of cognitive ability areas previously shown to be most consistently correlated with functional skills,
39 –
41 including attention, motor skills, verbal learning and memory, verbal fluency, and several aspects of executive functioning. A composite cognitive score was calculated by standardizing scores from the following measures into z-scores: animal naming, Wisconsin Card Sorting Test 64-card computerized version (WCST),
42 perseverative errors, WCST categories attained; Trail-Making Test,
43 parts A and B; Rey Auditory Verbal Learning Test (RAVLT),
44 learning trials 1–5, short delay free recall; the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)
45 version of the Boston Naming Test, constructional praxis assessment; WAIS, 3rd edition (WAIS-III),
46 digit span and letter-number sequencing subtests; and the Stroop Color Word Test interference condition.
47Adaptive life skills were assessed with the University of California at San Diego (UCSD) Performance-Based Skills Assessment Battery.
48 This performance-based measure is designed to directly assess functional skills competence among the severely mentally ill through the use of props and standardized skills performance situations. The comprehension/planning domain measures the patient’s ability to comprehend written material that describes recreational outings and then plan the activities and list appropriate items necessary to bring to the outings. In the finance domain, the patient must count out given amounts from real currency, make change, and fill out a check to pay a utility bill. The communication domain involves a series of role play situations that require the patient to make emergency calls, call directory assistance to request a telephone number, call the number, and then reschedule a medical appointment. In the transportation/mobility domain, patients use information from bus schedules and maps to determine appropriate fare, state telephone numbers to answer relevant questions, decide which map to use to get to a certain location, and determine the appropriate route and transfers to reach a destination. For the purposes of these analyses aimed at reducing the number of total correlations, the UCSD Performance-Based Skills Assessment Battery total score was used as the dependent variable.
The Social Skills Performance Assessment (SSPA)
49 is a performance-based measure of social skills that was created for use with schizophrenia patients. After a brief practice, the patients initiate and maintain a conversation for 3 minutes in each of two situations: greeting a new neighbor and calling a landlord to request a repair for a leak that has gone unfixed. These sessions were audio taped and scored by a trained rater who was unaware of diagnosis (patient or healthy comparison subjects) and all other data. Dimensions of social skills scored include fluency, clarity, focus, negotiation ability, persistence, and social appropriateness. These raters were trained to the gold standard ratings of the instrument developers (ICC=0.86), and high interrater reliability was maintained at 3 months (ICC=0.87). The mean of the ratings on these variables across the two measures was used in this study.
Real World Functional Behavior
The Specific Level of Function Scale, a 43-item observer-rated report of a patient’s behavior and functioning, was used to assess the following domains: interpersonal relationships (e.g., initiating, accepting and maintaining social contacts, effectively communicating); socially acceptability (e.g., verbal and physical abuse, repetitive behaviors); participation in community activities (e.g., shopping, using the telephone, paying bills, use of leisure time, use of public transportation); and work skills (e.g., employable skills, level of supervision, punctuality). For all participants in this study, ratings were provided by a caseworker or other clinician who indicated that they knew the patient at least “very well” on the Specific Level of Function Scale’s 5-point Likert scale. The scale has excellent interrater reliability, factorial validity, and internal consistency
36 and has been previously shown to be related to neuropsychological performance and scores on functional capacity measures.
50,
51 The clinicians providing ratings were unaware of all other aspects of the assessment.
Statistical Analyses
Our statistical analyses were employed to examine the prevalence and severity of depression, the validity of the BDI as an assessment tool in schizophrenia, and the criterion validity of ratings of depression. Since previous reports with the BDI in other populations have revealed a multifactorial structure, we were interested in examining the total score as well as factors that emerged from principal components factor analysis. We examined the prevalence and severity of depressive symptoms (in total and by the derived domains) with frequency analysis. We used Pearson’s correlation coefficients to examine the construct validity of the total scale and its domains. A measure has good construct validity if it converges with other measures purported to assess the same domain and diverges from other measures that assess different domains. Convergent validity was assessed by examining the association of BDI scores with clinically rated depression. Divergent validity was assessed by examining the association of BDI scores with other features of the illness including negative symptoms, positive symptoms, anxiety, and cognition. To examine the functional meaningfulness of the BDI total and domain scores, we examined their correlates with functional performance in the real world.
RESULTS
Frequency Analysis
Beck Depression Inventories (BDI) were completed by 230 of 240 schizophrenia patients who had other data in the longitudinal study. Ten patients discontinued testing prior to completing the BDI-II. The mean total score on the BDI-II was 11.3 (SD=9.6; range=0–50). The data were distributed with moderate, but acceptable, positive skew (1.2) and positive kurtosis (1.7). Thus, the data were not transformed. As displayed in
Figure 1, nearly 50% of patients endorsed what is conventionally considered at least mild depression, with nearly 20% endorsing moderate to severe depression.
Total Score Reliability
The BDI-II had good internal consistency (Cronbach alpha=0.88). However, low squared multiple correlations were observed for the irritability (0.15) and weight loss (0.20) items.
Data Reduction
Principal components factor analysis (PCFA) with varimax rotation was performed with the 21 items of the BDI-II. Principal components extraction estimated five components and absence of outliers, singularity, or multicollinearity. Bartlett’s test of sphericity (χ
2 =1517, df=210, p<0.001) and the Kaiser-Meyer-Olin measure of sampling adequacy (0.895) indicated excellent factorability. The variables were all well defined by the factor solution (all communalities >0.39), and five components had eigenvalues greater than 1.0. However, the fourth and fifth components were poorly defined as only two items (weight loss and appetite loss) loaded on the fourth component, and only the irritability item loaded on the fifth component. Therefore, a second principal components factor analysis was conducted with three components requested. Irritability, weight loss, and loss of interest were further deleted from this analysis as they had low communality (<0.11). Bartlett’s test of sphericity (χ
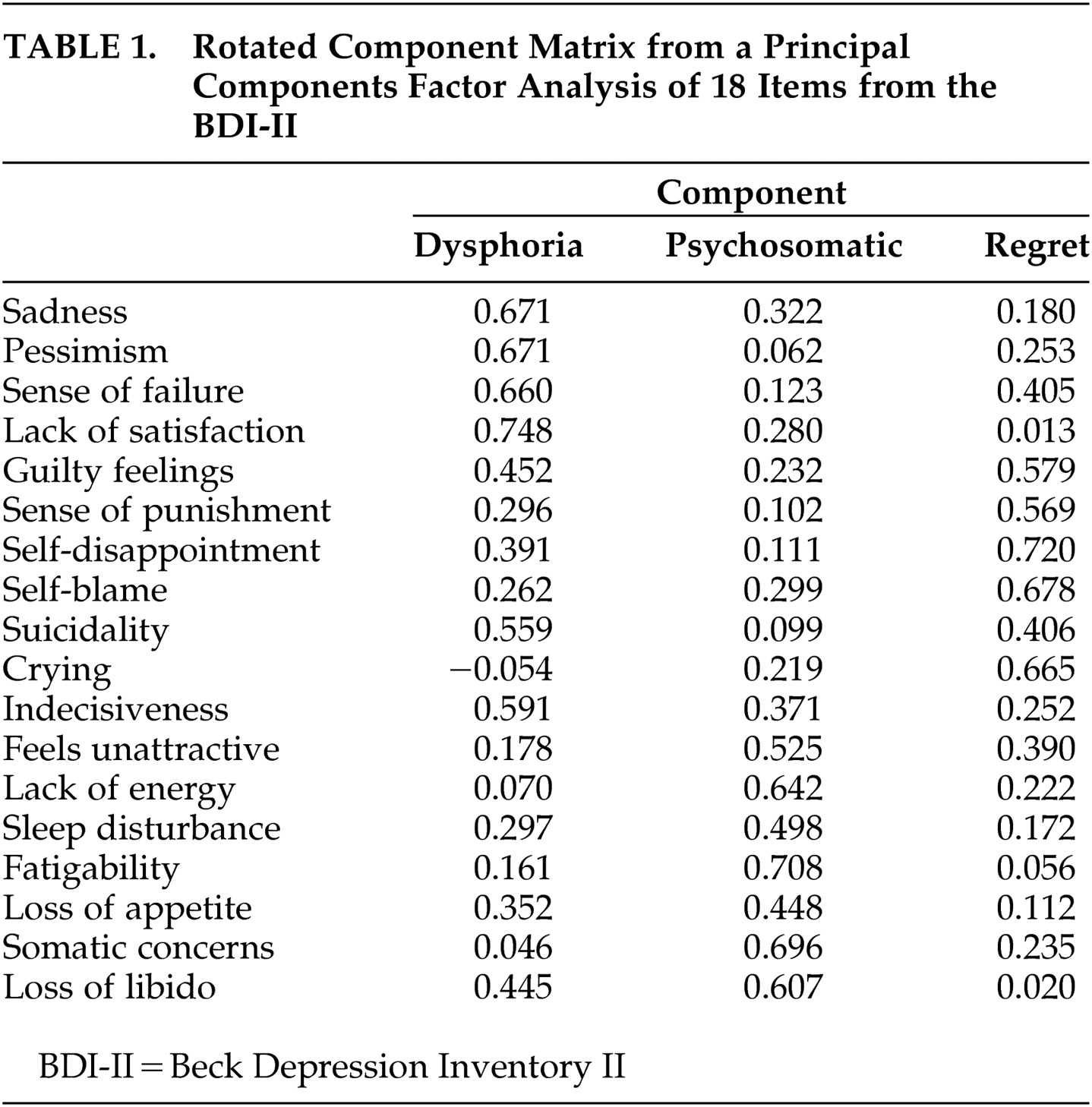
2 =1324, df=153, p<0.001) and the Kaiser-Meyer-Olin measure of sampling adequacy (0.9) indicated excellent factorability of this final three-component solution. Based on the individual items with the highest loadings on each component, these domains were named dysphoria (six items), psychosomatic (seven items), and regret (five items). See
Table 1 for the rotated factor score matrix with each component’s highest loading items highlighted.
Domain scores were created by summing the items that had primary loadings for each component. This summative procedure results in more meaningful domain scores than the regression-based approaches because it generates easily replicable domains for future studies and use in clinical practice and is acceptable given the items’ equivalent standard deviations.
52 Severity ratings for these domains were calculated by using the same percentage of the total possible score as conventional ratings for the total score. Thus, for the dysphoria domain to be rated as mild, its total must be between 23% and 32% of its total possible score, similar to the score of 15–20 of 64 on the total score. As displayed in
Figure 1, the distribution of the severity of these domain scores did not mimic the total score distribution.
Domain Score Reliability
The summed component scores were tested for internal consistency with Cronbach alpha. Despite having fewer items than the total score, which may artificially deflate the statistic, comparably good consistency was observed for the dysphoria (0.82), psychosomatic (0.78), and regret (0.75) domains. All squared multiple correlations were greater than 0.20.
Construct Validity
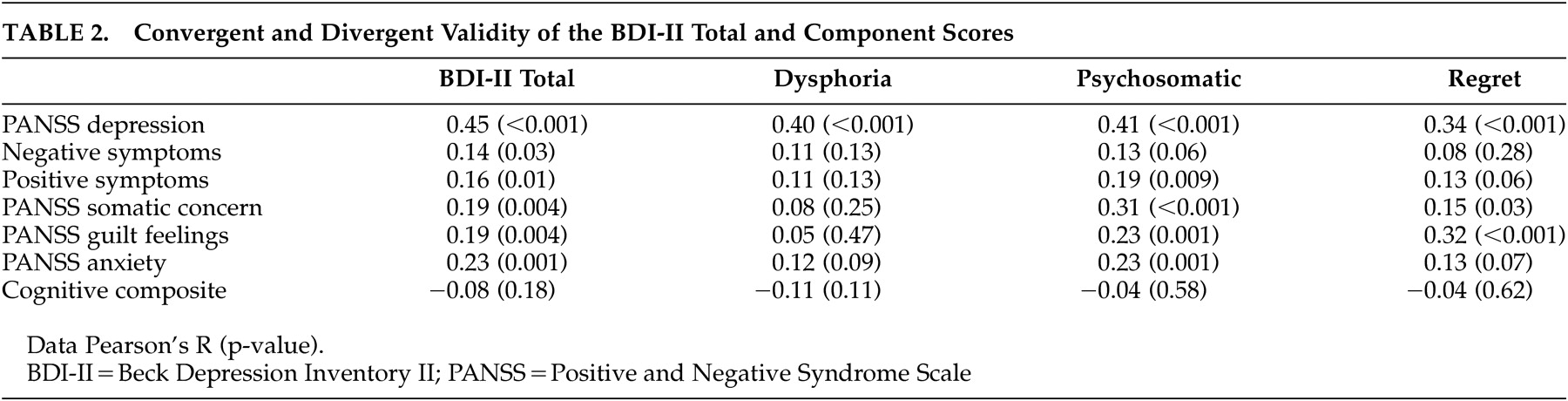
Convergent and discriminant validity were assessed by examining correlations among the total and domain scores of the BDI and corresponding ratings from the Positive and Negative Syndrome Scale (
Table 2 ). The BDI Total and all three domains evidenced convergent validity. All were significantly correlated with clinically rated depression (p<0.001 in all cases). The psychosomatic domain was correlated with the clinically rated somatic concern, and the regret domain was correlated with clinically rated guilt. However, only the dysphoria and regret domains provided evidence for discriminant validity, with small and nonsignificant correlations with clinically rated negative and positive symptoms, somatic concern, guilt, and anxiety. The BDI total score showed poor discriminant validity by virtue of significant correlations with negative symptoms and anxiety. The psychosomatic domain failed to diverge from positive symptoms and anxiety. Thus, adequate construct validity was found for the dysphoria and regret domains, but not for the BDI total score or the psychosomatic domain.
Correlates With Skills and Behavior
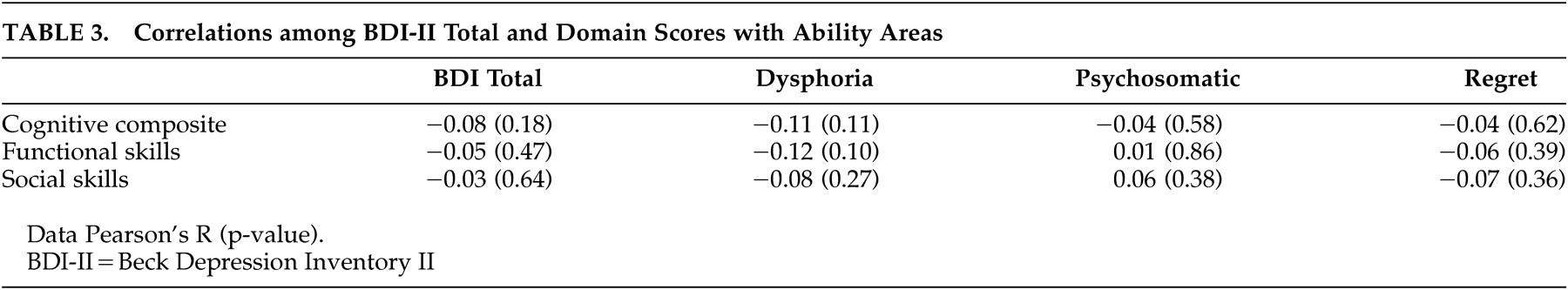
Pearson correlation coefficients were used to examine the relationship of the BDI-II total score and domain scores with functional skills and real world functional behavior. As displayed in
Table 3, neither the total score nor domain scores were significantly associated with cognitive, functional, or social performance.
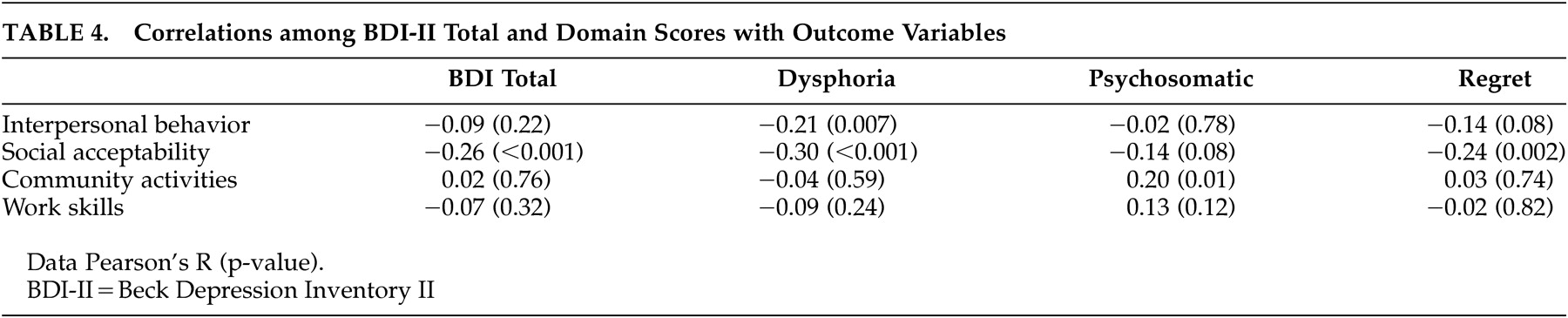
In contrast to the lack of correlation with neuropsychological competence and functional capacity, significant associations were found with real world behavior. The dysphoria domain was most consistently related to several elements of functional disability, with significant inverse relationships between it and interpersonal behavior and socially acceptable behavior. An unexpected positive relationship was observed, with greater psychosomatic symptoms associated with more community participation. See
Table 4 for the relationships among BDI and outcome measures.
DISCUSSION
As previously reported by other investigators,
2,
53 our study demonstrates that depression is a highly prevalent symptom in older outpatients with schizophrenia. In this patient population, depression is more likely to be mild in severity, although a substantial minority of patients reported more severe mood disturbance. Consistent with this finding, Zisook et al.
54 previously reported that in elderly patients with schizophrenia, mild (and even subsyndromal) depression was highly prevalent and was associated with functional impairment of clinical importance. The negative impact of even mild forms of depression on functional status in populations at increased risk for disability, such as elderly people without schizophrenia attending primary care treatment
55 and older patients with schizophrenia,
56 is usually underrecognized.
Few studies have examined the symptom structure of depression and associated symptoms in a population of older patients with chronic schizophrenia. In the present study the BDI-II was utilized as a measure of depression. The BDI-II is a 21-item self-rating scale developed from the original BDI, which was designed to measure the manifestations of depression in nonpsychotic populations
14 and has been previously validated in patient and healthy populations.
57,
58 The factor structure of this scale was examined for various populations including traumatic brain injury,
59 chronic pain,
60 and alcohol dependence.
61 However, the use of small sample sizes by these studies has prevented the extraction of stable factors, leading to skepticism in interpreting the BDI as multifactorial.
62 Conversely, our study included a large population of older outpatients with schizophrenia. Three stable components were extracted and two components (dysphoria and psychosomatic) were found to be significantly correlated with clinically rated depression, but unassociated with negative and positive symptoms, guilt, anxiety, and cognitive impairment. These results support the notion that two features of depression, as assessed with the BDI-II, are independent and functionally relevant features in schizophrenia.
When examining the total BDI-II score, the assessment of depression in schizophrenia appears to be at least partially a methodological artifact of negative symptoms. However, in our study, specific items of this scale were distinct from negative symptoms and other aspects of the illness. These items, representing mood disturbance, were not only statistically distinct, they also do not show definitional overlap with negative symptoms or cognitive impairment, suggesting good face validity. Thus, depression may be a distinct feature of the illness for some patients that is detectable with specific items that represent true mood disturbance. To reduce overlap with negative symptoms, the examination of depressive symptom treatment response in this disorder should be multifaceted.
Our findings support the notion that clinicians should be mindful of the potential, in patients with schizophrenia, for negative symptoms to influence ratings of depression, and should be aware that depressive symptoms in these patients might lead to functional limitations, particularly in the actual deployment of skills in the real world. Thus, for many patients with schizophrenia, depression may be an important treatment target for disability reduction.
Similar to our previous findings with the BDI-II total score in the same overall sample of subjects,
50,
51 the three domains had small nonsignificant correlations with cognitive and functional abilities, while severity of dysphoric symptoms was directly associated with impairment in two domains of real world behaviors. Thus, depression in schizophrenia, specifically self-reported mood disturbance, may be a postcompetence, rate-limiting factor in efforts to improve functional outcomes.
By avoiding the limitations of this study, future research will be able to provide further knowledge of the features of depression in patients with schizophrenia. Our sample consisted of older patients assessed after many years of chronic symptoms. The features of depression in this population may not be similar to the symptoms present in younger and more acutely ill patients. Furthermore, the features of depression in schizophrenia might be better determined by the use of a scale specifically developed for the assessment of depressive symptoms in this disorder such as the Calgary Depression Scale. A potential problem with the BDI-II is that it relies on a patient’s self-report. Patients with schizophrenia often have impaired ability to recognize their symptoms
63 and functional deficits.
21,
64,
65 Obviating this concern is the present finding that patient self-reported depression converged with clinician ratings. These clinician ratings, although based on an interview with the patient, also take into account observable signs as well as interviews with caretakers. Associated depressive symptoms in schizophrenia were found to correlate with real world disability, highlighting the need for elucidating the components of depression in this disorder to better treat the adverse impact of depressive symptoms in these patients. Despite limitations, our study is an initial step to discriminate discrete components of the depression disorder associated with schizophrenia.
Future work might also be able to address other mood symptoms associated with schizophrenia such as mania, obsessions, compulsions, and anxiety, particularly social anxiety, which is frequently observed in these patients.
Acknowledgments
This research was supported by NIMH Grant # MH 63116 to Dr. Harvey, the Mt. Sinai Silvio Conte Neuroscience Center (NIMH MH 36692; KL Davis PI), and the VA VISN 3 MIRECC. The authors thank Kushik Jaga and Brooke Halpern for assistance with data collection and technical aspects of the report preparation. Dr. Bowie has been a paid consultant to Pfizer, Inc., and received grant support from Pfizer, Inc., and Janssen, LTD. Dr. Harvey is a consultant for Pfizer, Inc., Janssen Medical Affairs, Sanofi-Aventis, Astra-Zeneca, Abbott Labs, Memory Pharmaceuticals, the Solvay-Wyeth group, and Merck, Inc; he is on the advisory board for Eli Lilly and Forest Laboratories.