Fifteen volunteers (nine men and six women, mean age=38.5 years, SD=12.9, range=22–63) gave written informed consent for this study, which was approved by an internal review board. They received financial compensation for time and travel. They were interviewed with the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (
3), did not meet criteria for any DSM-IV axis I or II disorder, had no history of psychiatric illness in first-degree relatives, were in good physical health, were not taking medication, and did not use any substances (besides nicotine in seven cases). Thirteen were employed, one was retired, and one was a student; 12 were living with a partner.
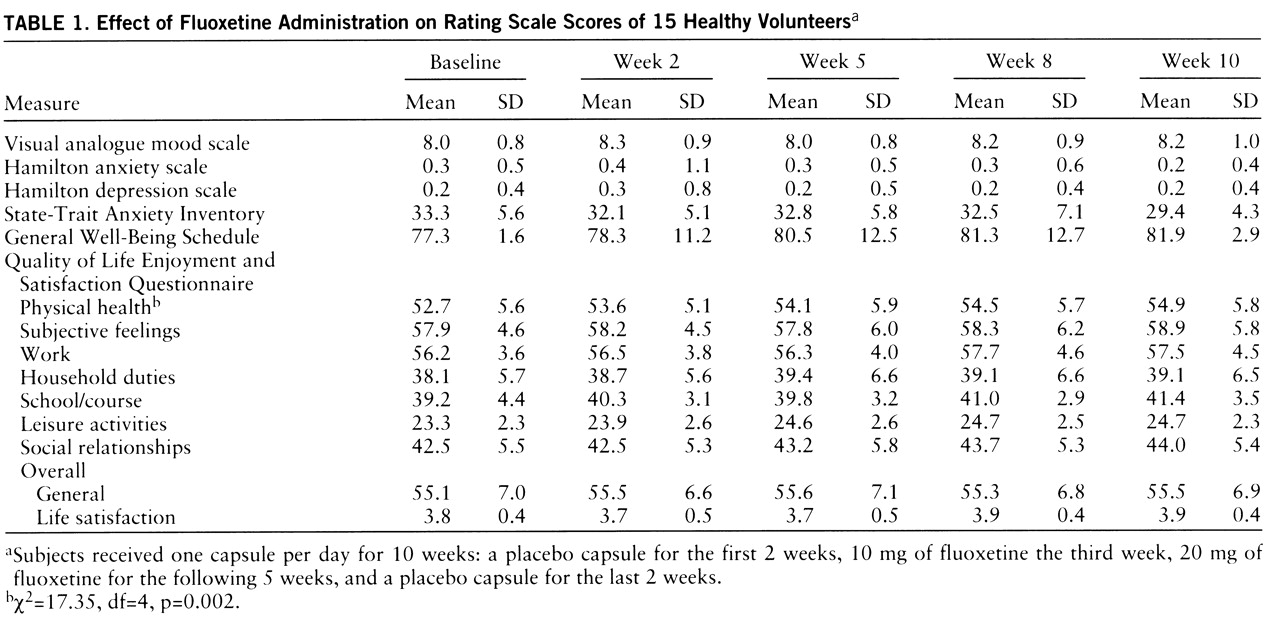
For 10 weeks the subjects were given one capsule per day: a placebo capsule for the first 2 weeks, 10 mg of fluoxetine the next week, 20 mg of fluoxetine for the following 5 weeks, and a placebo capsule for the last 2 weeks. They did not know for how long or when placebo would be administered. They were followed up in person after 2 weeks (end of first placebo phase), 5 weeks (after 3 weeks of receiving fluoxetine), 8 weeks (after 6 weeks of receiving fluoxetine), and after the final 2 weeks of placebo administration. They were assessed with the Hamilton anxiety and depression scales (
4) at each visit and filled in a 10-cm visual analogue mood scale (
5,
6), the State-Trait Anxiety Inventory (
7), the General Well-Being Schedule (
8), and the Quality of Life Enjoyment and Satisfaction Questionnaire (
9). Visual analogue mood scales have been extensively used for the assessment of subjective phenomena, including drug effects (
5), and have been shown to be sensitive to individual differences in nonclinical settings (
6). The General Well-Being Schedule (
8) generates a score that reflects overall well-being and scores on six subscales. The Quality of Life Enjoyment and Satisfaction Questionnaire (
9) provides a sensitive measure of the enjoyment and satisfaction experienced by the subject in various areas of daily functioning. The Comrey Personality Scales (
10) provide scores on eight personality dimensions, a validity check for random scoring, and a response bias check. These scales were administered at baseline and after 6 weeks of fluoxetine administration. Information on adverse effects was obtained through a general inquiry and by a checklist at each visit and by telephone at the end of each week in which the subject was not seen in person.
Because the data were not normally distributed, Friedman's nonparametric analysis of variance with repeated measures was employed to evaluate changes in the rating scale scores over the course of the trial. If significant, the Wilcoxon signed ranks test was applied to compare time points. Bonferroni correction (α=0.05) was applied.