Cross-sectional studies (
1–
3) and prospective studies (
4–
8) in both HIV-positive and at-risk HIV populations estimate the lifetime prevalence of mood disorders as 22.1%–61.0% (
4,
6–
8), with current (1 or 2 months) prevalence ranging from 0% to 18.4% in HIV-seropositive populations and from 0% to 9.1% in HIV-seronegative populations (
1–
8). While depression-like syndromes are difficult to diagnose in the context of medical illness, these rates are elevated in comparison with estimates in two community samples (
9,
10) of lifetime diagnoses (5% and 17%, respectively) and current diagnoses (3% and 10%, respectively) of major depression.
Depression in patients with HIV infection has been effectively treated in open trials with fluoxetine (
14–
16), imipramine (
17), sertraline (
18), fluvoxamine (
19), methylphenidate (
20), desipramine (
20), and testosterone (
21). All of these studies have demonstrated a response rate similar to that seen in medically healthy depressed patients. Imipramine, fluoxetine, sertraline, and testosterone demonstrated response that was unrelated to the severity of immunosuppression (
15,
18,
21,
22). Of these, only imipramine has been investigated in a randomized, placebo-controlled trial (
22). A high rate of imipramine discontinuation in that trial, coupled with open-trial reports of mild and infrequent side effects of fluoxetine (
15,
16) and sertraline (
18) (but not fluvoxamine [19]) in depressed HIV-positive patients, suggests that selective serotonin reuptake inhibitors (SSRIs), although not more efficacious, may be more tolerable and have greater overall effectiveness in this population (i.e., a higher proportion of treated patients who benefit). While methylphenidate has shown a response rate similar to and consistent with that in other depression-like syndromes in the context of medical illness (
20), it is often useful as an adjunctive medication and may show beneficial effects in the presence of cognitive deficits.
METHOD
One hundred thirty-two HIV-positive persons were screened for study entry from outpatient psychiatric and medical clinics and through advertisements. Of these, 57 individuals were excluded for withdrawal of consent or poor compliance (N=20), a diagnosis of bipolar disorder (N=8) or substance use (N=12), current antidepressant treatment (N=9), failure to meet diagnostic criteria (N=5), and severe medical illness (N=3). The 75 subjects enrolled in the study had a mean age of 36.0 years (SD=7.5), and their mean number of years of education was 13.4 (SD=2.3). All subjects gave written informed consent.
Exclusion criteria included alcohol or substance abuse within the last month, confirmed by urine drug testing (48% had a history of alcohol use; 29%, marijuana use; and 32%, cocaine use), prior diagnosis of organic brain syndrome, dementia, severe concurrent HIV-related physical illness, fewer than 12 years of education, high suicide risk, or a history of bipolar disorder, traumatic head injury, or psychosis.
After experiencing a high attrition rate (63%) during enrollment of the first 11 patients, we provided subjects with a $10 payment per visit to cover the cost of travel and lunch, which was found to be burdensome for many of these patients living on limited income. This payment was not offered until after the subject had been screened into the protocol. This resulted in a nonsignificant reduction of the dropout rate (to 53%) for the remaining patients entered (N=64).
Subjects were randomly assigned to blind treatment with paroxetine (N=25), imipramine (N=25), or placebo (N=25). Paroxetine was started at 10 mg/day and increased to 20 mg/day by the first week and 40 mg/day by the second week if tolerated. Imipramine was started at 50 mg/day and increased to 100 mg/day by the first week and 200 mg/day by the second week if tolerated. After 2 weeks, dosages were not increased for either group and were only decreased in relation to side effects or medical illness. Assessments were made at baseline and weeks 2, 4, 6, 8, and 12. At 6 weeks, to accommodate human subject committee concerns, subjects not showing improvement (reduction in Hamilton Depression Rating Scale score of at least 25%) were withdrawn from the blinded protocol (they were considered as dropouts due to lack of efficacy) and treated with the other antidepressant in an open-label fashion for the remainder of the study (6 weeks).
Ratings and Assessments
Current diagnoses were assessed with a modified version of the Structured Clinical Interview for DSM-III-R (SCID) (
24). Subjects with a DSM-III-R diagnosis of major depression according to the SCID interview and a score of 18 or more on the 21-item Hamilton Depression Rating Scale (
25) entered the trial. In addition to repetitive assessments with the Hamilton depression scale, scores on the Hamilton Anxiety Rating Scale (
26), the Clinical Global Impression (CGI) scale (
27), the grooved pegboard (
28) (to assess basic neuromotor functioning), and the Brief Symptom Inventory (
29) were used at baseline to provide a more extensive clinical description of the study participants. The SAFETEE general inquiry (
30) was used to retrieve unbiased medication-related sided effects volunteered by patients at every visit in response to the general screening item “Have you had any health-related or physical problems since your last visit?” If patients described a new complaint, they were asked to describe it in more detail to determine whether it was new or preexisting. Preexisting symptoms or complaints were not scored as side effects. A checklist was used at the termination of the study to classify reported side effects into categorical groups. All ratings were made by one of two authors (K.B. and A.J.E.), who were blind to study drug assignment. Interrater reliability (N=25) was high, with good intra~class correlations (for Hamilton anxiety score, 0.95; for Hamilton depression score, 0.97).
Finally, because of the high frequency of subsyndromal (somatic) symptoms of HIV infection overlapping with symptoms of depression (
31–
33), we developed two nonsomatic scales, one from the Hamilton anxiety scale (items 1–6 and 14) and the other from the Hamilton depression scale (items 1–3, 7–10, and 17–21), to eliminate symptoms potentially due to medical illness. The mean internal consistency over all assessment periods for these subscales was good (for nonsomatic anxiety, α=0.75; for nonsomatic depression, α=0.67).
CD4 cell counts and percents were analyzed at the University of Washington laboratory. HIV-related symptoms (e.g., chronic diarrhea, thrush) and AIDS-defining symptoms (e.g.,
Pneumocystis carinii pneumonia) were assessed at weeks 0 and 12 with a checklist based on 1993 Centers for Disease Control (CDC) AIDS-defining conditions (
34). Subjects were separated for analysis into three groups on the basis of the 1993 CDC criteria: HIV-positive, asymptomatic; HIV-positive, symptomatic; and AIDS.
Data Analysis
The clinical, medical illness, and demographic comparability at baseline of the three treatment groups and the groups completing and not completing the trial were assessed by means of one-way analysis of variance (ANOVA) for continuous data and the chi-square test or Fisher's exact test for categorical measures.
Response to treatment was analyzed with repeated measures ANOVAs (with drug type as the between-groups factor and time as the within-subject factor) using the continuous outcome variables (scores on the Hamilton depression scale, nonsomatic Hamilton depression items, the Hamilton anxiety scale, nonsomatic Hamilton anxiety items, and the CGI). The analyses were performed separately for the intent-to-treat study group, with the last observation carried forward for the patients who completed 4 weeks of the trial, and for those who completed the entire trial. In the event of a significant drug effect or drug-by-time interaction, post hoc ANOVAs were performed. Exact Greenhouse-Geisser adjustments to the degrees of freedom were used to account for violations in compound symmetry in repeated measures analyses. In addition, we used CD4 cell count and percent and number of HIV-related and AIDS-defining symptoms as covariates. If these were not statistically significant, we dropped them from the analyses and recalculated the ANOVAs.
Using chi-square analysis, we also assessed the proportion of responders in each group. We defined partial response as a 50% decrease in Hamilton depression score (at week 12) from baseline and full response as a Hamilton depression score of less than 8 (
35). For the CGI, response was defined as a final CGI rating of 1 (very much better) or 2 (much better). Responder analyses were performed for weeks showing significant drug-placebo differences by the ANOVAs for both the intent-to-treat and completer groups.
The frequency of side effects was compared between groups with the chi-square test or Fisher's exact test for all patients who received at least one dose of active drug. Any reported side effects occurring up to and including the 6-week visit were included, regardless of their persistence. Dropout rates due to side effects, lack of efficacy, or other causes were also compared by chi-square analysis. We included CD4 cell count and percent and HIV-related and AIDS-defining symptoms as covariates. All reported p values are for two-tailed tests of significance.
RESULTS
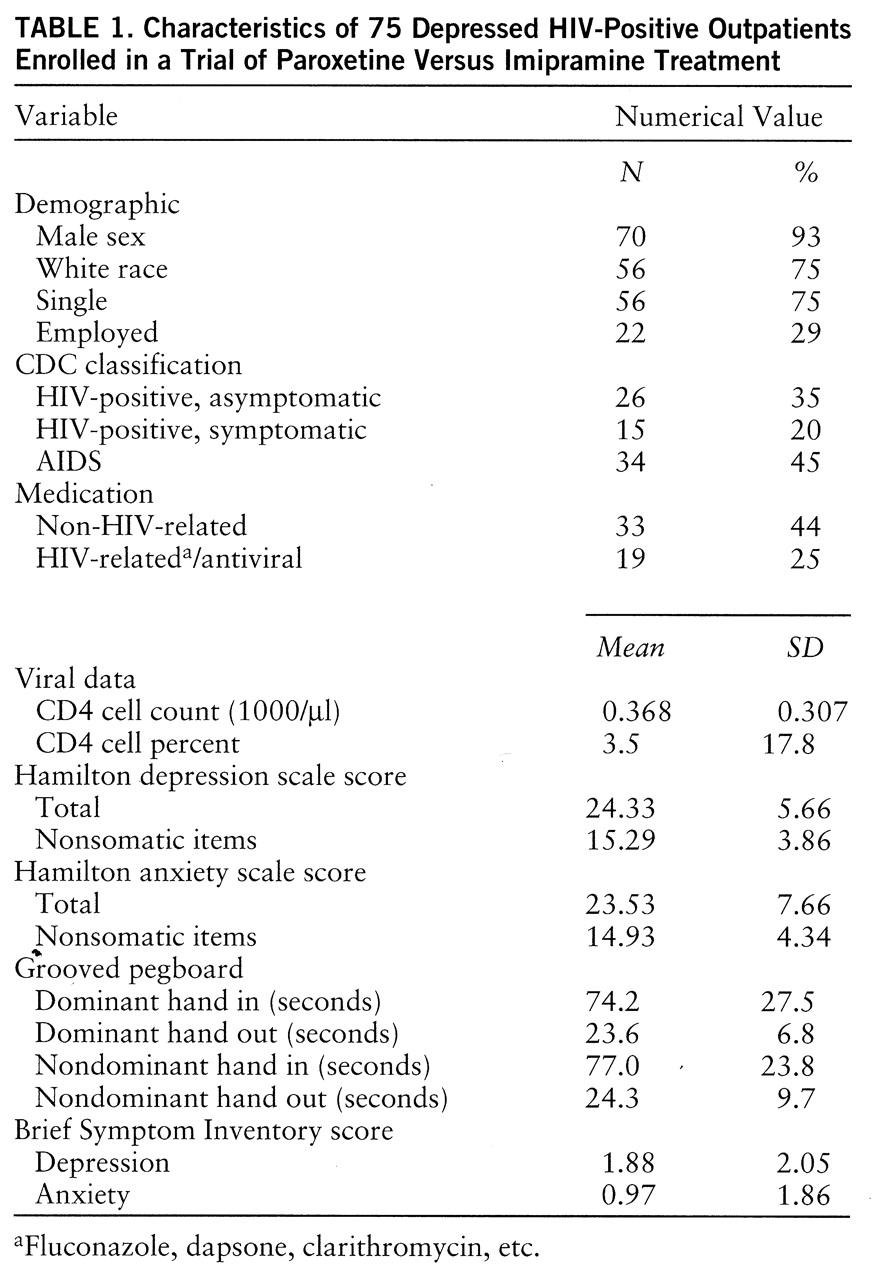
Because there were no significant differences between treatment groups or between subjects who dropped out and those who completed the study protocol, a summary of the demographic, medical, psychiatric, and other clinical characteristics of the entire study group (N=75) is presented in
table 1. Sixty percent (N=45) had had a prior depressive episode, all but three (4%) had been depressed for less than a year, and 24 (32%) had comorbid dysthymia (double depression). Of those who entered the trial, 45% (N=34) had previously received antidepressant treatment.
Active treatment for at least 4 weeks was received by 56 (75%) of the 75 subjects (placebo, N=22; paroxetine, N=18; imipramine, N=16), while 34 (45%) of the 75 subjects completed the entire 12-week trial (placebo, N=13; paroxetine, N=11; imipramine, N=10). Reasons for dropout included adverse reactions/side effects (placebo, N=6; paroxetine, N=5; imipramine, N=12), worsening HIV illness (placebo, N=0; paroxetine, N=4; imipramine, N=1), lack of efficacy (placebo, N=6; paroxetine, N=3; imipramine, N=1), and other (placebo, N=0; paroxetine, N=2; imipramine, N=1).
All paroxetine-treated patients received at least 20 mg/day (4-week mean dose=33.9 mg/day, SD=9.2), while 44% (N=11 of 25) of the intent-to-treat imipramine group and 69% (N=11 of 16) of the imipramine-treated patients who completed the study received at least 150 mg/day (4-week mean dose=162.5 mg/day, SD=53.2).
Paroxetine and imipramine demonstrated similar antidepressant efficacy as measured by each of the outcome variables. For this reason, analyses using the Hamilton anxiety scale and the nonsomatic versions of both the Hamilton anxiety and Hamilton depression scales are not presented. When CD4 cell count and percent and number of HIV-related and AIDS-defining symptoms were used as covariates, there was no statistically significant difference, and therefore they were dropped from the analyses and ANOVAs were recalculated.
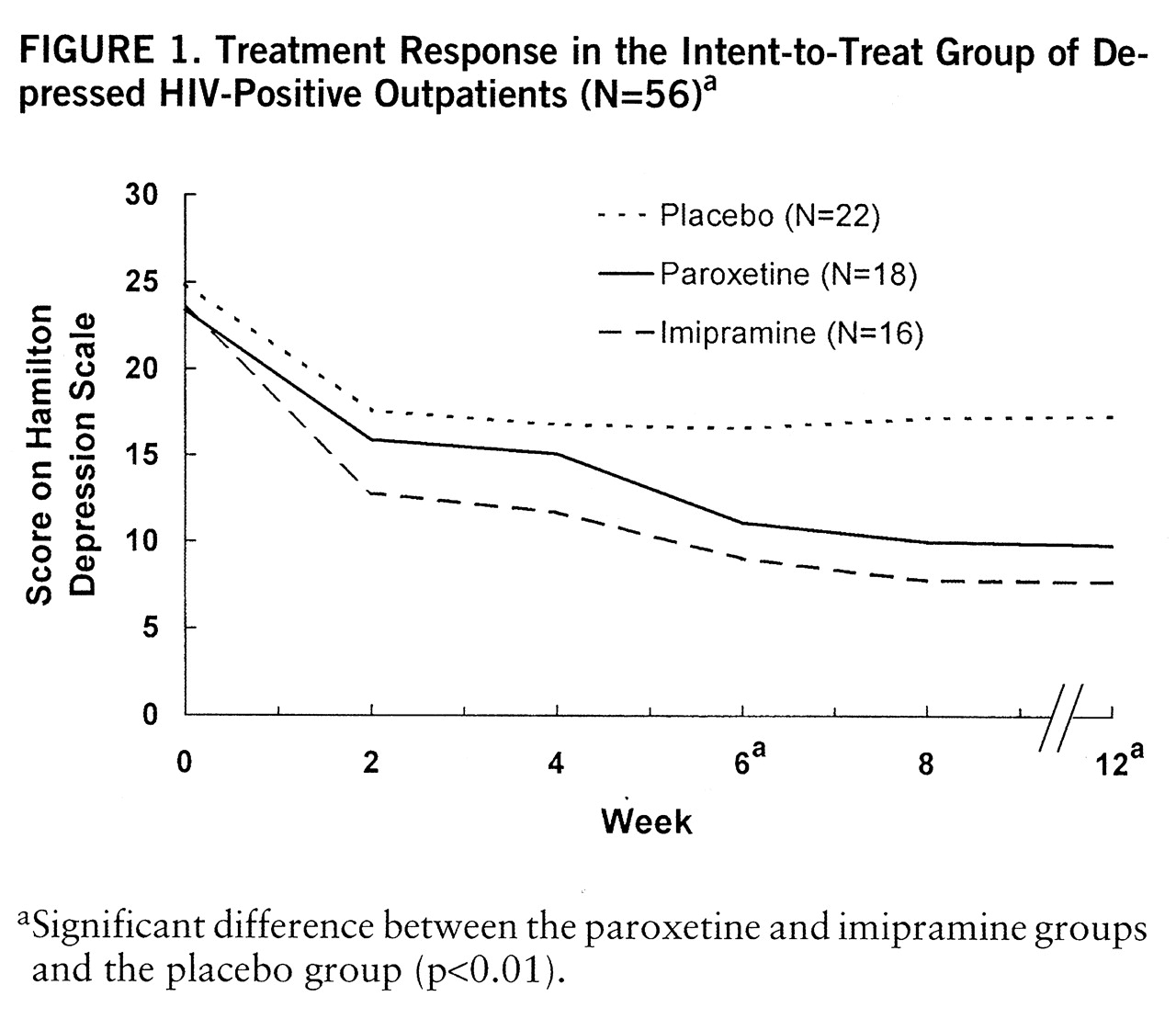
Intent-to-treat analysis showed a significant drug-by-time interaction (F=2.65, df=5.86,155.16, p<0.02), drug effect (F=6.91, df=2,53, p<0.002), and time effect (F=51.04, df=2.93,115.16, p<0.001) for Hamilton depression scale score as well as a drug effect (F=9.09, df=2,53, p<0.001) and time effect (F=3.54, df=2.75,145.98, p<0.02) for CGI score. Post hoc analysis showed significant superiority of both active drugs over placebo at week 6 (F=5.74, df=2,53, p<0.01), week 8 (F=2.53, df=2,53, p<0.01), and week 12 (F=8.69, df=2,53, p<0.01) for both measures (
figure 1).
Analysis of the response of the subjects who completed the trial showed significant effects on Hamilton depression score for drug (F=3.68, df=2,31, p<0.04) and time (F=61.92, df=3.69,114.51, p<0.001) and also on CGI score for drug (F=4.80, 2,30, p<0.02) and time (F=3.08, df=3.07,95.04, p<0.03). Post hoc analysis revealed less consistent effects. Both drugs were superior to placebo at week 8 (F=7.70, df=2,31, p<0.01) for Hamilton depression score, whereas only imipramine was superior to placebo at week 12 (F=4.29, df=2,31, p<0.05) for Hamilton depression score and CGI score.
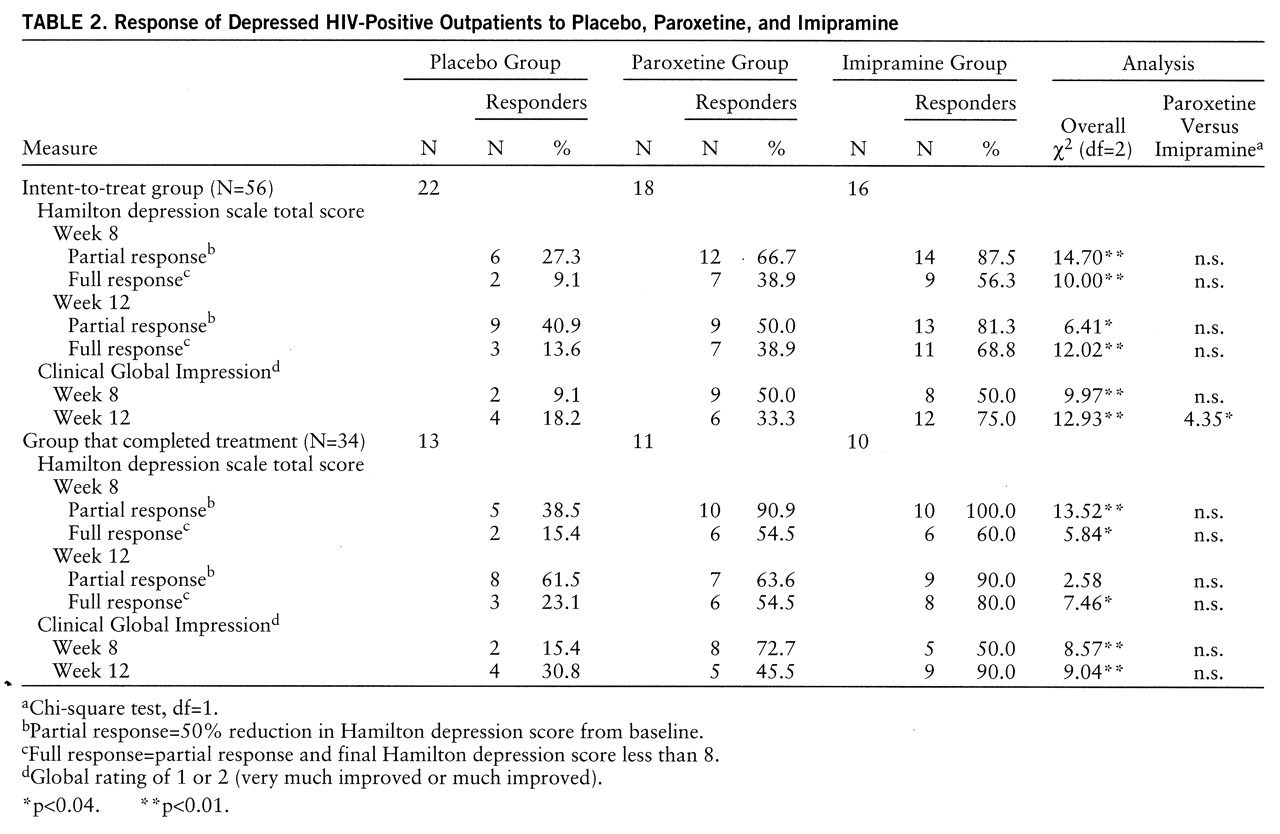
Table 2 shows response rates in the intent-to-treat and completer groups. Intent-to-treat analysis showed higher response rates for both active drugs compared to placebo at week 8 and week 12 according to all three response criteria. Completer analysis also showed higher response rates for both drugs compared to placebo at weeks 8 and 12, except for partial response rates at week 12, where a high placebo response rate (61.5%) obviated drug-placebo differences. While response rates appear numerically higher for imipramine than for placebo in many of these 12 comparisons, these rates were not significantly different from each other except for CGI response at week 12 in the intent-to-treat group.
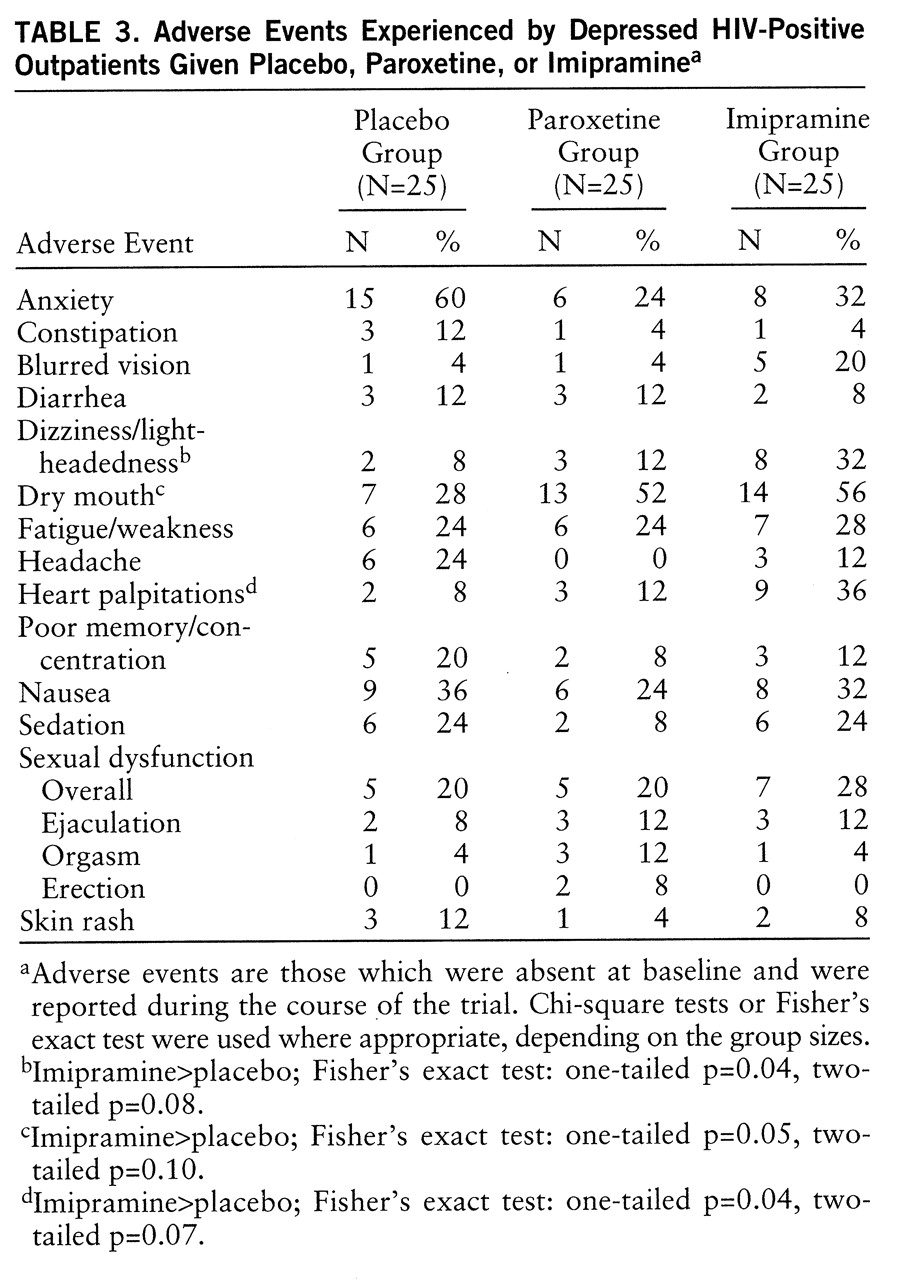
A summary of the most commonly reported adverse events (>5% and not present at baseline) is shown in
table 3. Twenty-three patients (31%) discontinued treatment because of adverse events. A higher proportion of the 25 imipramine-treated patients (48%, N=12) than of the 25 paroxetine-treated patients (20%, N=5) and the 25 placebo-treated patients (24%, N=6) discontinued treatment because of adverse events (χ
2=6.98, df=2, p=0.03). The mean number of adverse events was 3.6 (SD=2.2) in the placebo group, 3.1 (SD=2.9) in the paroxetine group, and 3.9 (SD=2.9) in the imipramine group (nonsignificant difference). Dry mouth, dizziness/postural hypotension, and palpitations were significantly more prevalent with imipramine than with both paroxetine and placebo, whereas dry mouth was more prevalent with imipramine than with placebo. The rates of sexual dysfunction in this study group were similar for both active drugs and placebo (
table 3), although erectile dysfunction was observed only in the paroxetine-treated patients, albeit at a low rate (8%).
Of the 10 patients removed from the study at week 6 for lack of efficacy, seven (six who had been taking placebo and one who had been taking imipramine) were treated openly with paroxetine and three (who had been taking paroxetine) were treated with imipramine. Partial and full response rates for paroxetine at week 12 (after 6 weeks of treatment) were 71% (N=5) and 57% (N=4), respectively. Partial and full response rates for imipramine were 33% (N=1) and 0% (N=0), respectively.
There was no apparent relationship between response to antidepressants and HIV status, symptom severity, lifetime psychiatric history, history of or treatment for depression, or chronicity of depression.
DISCUSSION
Our findings document the effectiveness of both imipramine and paroxetine compared with placebo in depressed patients with HIV infection. Both intent-to-treat and completer analyses of mean differences between treatment groups suggest that the two antidepressants are comparably effective, as does chi-square analysis of response rates in these groups. Numerically higher response rates for imipramine than for paroxetine were significantly different in only one of 12 analyses. Furthermore, in the small group of nonresponders, paroxetine had a higher response rate than imipramine, although this may be because all subjects who did not respond to placebo received paroxetine, while all of those who did not respond to paroxetine (perhaps a more treatment-refractory group) received imipramine.
Our findings are consistent with a recent meta-analytic summary of HIV-depression drug treatment trials reporting similar response rates for SSRIs and imipramine (
36). Unfortunately a disproportionate number of our paroxetine patients (N=4, 16%) had to withdraw because of worsening HIV illness (
Pneumocystis carinii pneumonia, lymphoma, etc.) compared with placebo patients (N=0) and imipramine patients (N=1), which may have affected the response rate in this group and contributed to nonsignificant numerical differences. The high attrition rate in this study points out the hazard of small-group studies. Particularly in this study group, where nearly half of the subjects had AIDS, both nondrug and nondepression factors due to the high medical and psychosocial burden of HIV illness could influence outcome, and this influence may operate disproportionately in small-group studies.
The data suggest that imipramine is less well tolerated than paroxetine. Imipramine-treated patients showed a substantially higher dropout rate due to side effects than patients taking paroxetine (48% versus 20%) and more statistically significant side effects than patients taking placebo. However, there was a substantial level of dropout for both drug groups, and this may be related to the burden of HIV illness itself. The gap in side effects between the two drugs was not as large as expected, suggesting that certain side effects (constipation, sedation) may have been therapeutic for some HIV patients (i.e., those with symptoms of diarrhea and insomnia). Our expectation of a higher rate of paroxetine-induced sexual dysfunction (based on our observations before breaking the blind) (
37) was not borne out, as the 17 patients complaining of these side effects appeared to be equally distributed among the three treatment groups.
These side effect observations, when taken together with the efficacy analyses, suggest that imipramine is at least as effective as paroxetine for HIV-positive patients who are able to tolerate it, but that some patients may experience intolerable side effects, and this group may be difficult to keep in a controlled clinical trial. If the response rates for the two active drugs are multiplied by the proportion able to tolerate them (80% for paroxetine and 46% for imipramine), the overall effectiveness (ultimate rate of response in those initially given the drug) of paroxetine is greater.
This study had a number of limitations: 1) a high dropout rate, which was likely due to the burden of study participation for HIV patients, the option of discontinuing study medication at week 6, a higher Hamilton depression score at entry (mean=24.3) than that reported in other HIV-depression studies (mean baseline score=16–18) (
36), and a design in which patients were seen weekly (as has been done in other studies), which might have improved adherence to medication regimens; 2) lower doses of imipramine than those used in studies of nonmedically ill depressed patients (although these doses are more likely appropriate for this population); 3) a high percentage of male subjects (93%), which although it is consistent with both the demographic characteristics of nonintravenous-drug-abusing HIV patients and prior HIV depression studies, prevents extrapolation of results to female HIV patients; 4) a high response to placebo; and 5) the difficulty of assessing depression-like syndromes in the context of medical illness and the difficulty of distinguishing “mood disorder due to a medical illness” from major depression.
However, despite the dropout rates, the relatively small intent-to-treat cell sizes, and the added burden of HIV illness, statistical evidence of the efficacy of both active drugs and the greater tolerability of paroxetine was observed. In addition, this study further confirms previous reports that have described the absence of any relationship between medical status (CD4 cell count and percent or CDC-defined stage of illness) and treatment outcome.
These findings highlight the need for studies with larger numbers of depressed HIV patients, where multiple medications can be compared and more female patients can participate, although the difficulties in recruitment and retention of HIV patients for studies like this may make this difficult. On the basis of a recent review (
36), it appears that alternative treatments for depression such as psychostimulants and androgens, which may work more rapidly and provide added benefits for energy level and physical activity, must be included in such comparative trials. Given that almost one-half of our study group was medically ill with AIDS, it is important to note that present-day pharmacotherapy can effectively treat major depression in HIV patients, regardless of disease severity, and potentially minimize HIV mortality.