The effect of sex on functional neuroimaging studies has received increasing attention. Although findings have not always been consistent, women have generally displayed higher regional cerebral glucose metabolism than men at rest (
1) and during activation (
2,
3) as well as higher regional (
4) and global (
5) cerebral blood flow, which may vary as a function of cognitive state (
5).
Functional magnetic resonance imaging (MRI) is a high-resolution, minimally invasive tool that has been used to demonstrate sex differences in the functional organization of language centers (
6). We investigated the hypothesis that sex differences may also be detected by using a noncognitive, primary sensory activation such as the well-characterized, robust blood-oxygenation-level-dependent (BOLD) functional MRI response to photic stimulation (
7).
METHOD
Subjects. Sixteen healthy right-handed adult subjects were studied. Eight subjects were women (mean age=24.8 years, range=21–36), and eight were men (mean age=24.5 years, range=20–29). Each subject had normal results on neurological and psychiatric examinations, normal visual acuity, and normal conventional brain MRI. No subject reported a history of mood abnormality or treatment with psychotropic medication. The study was approved by the McLean Hospital Institutional Review Board, and written informed consent was obtained from each subject.
Imaging protocol. Imaging methods have been described elsewhere (
7). For BOLD imaging, gradient echo-echo planar images were collected from a single 10-mm slice through the visual cortex by using a surface coil (TR=1 second, TE=40 msec, flip angle=66°, one average, matrix=64×128, field of view=20×40 cm, in-plane resolution=3×3 mm). The 256-second acquisition period consisted of 4 epochs of 30 seconds of darkness alternating with 30 seconds of binocular photic stimulation, provided by light-emitting diode goggles at 8 Hz and with an intensity of 20 candelas/m
2.
Image analysis. Images were processed by using two methods: an interactive regional signal profile method and a semiautomated statistical mapping method (
7). Using the regional signal profile method, two analysts blind to subject group viewed the time-series image set with an interactive regional signal profile tool, allowing determination of MR signal change over time in a 6×6-mm region of interest placed in the right and left primary visual cortices of each subject. The mean percent MR signal with activation, termed BOLD signal response, was determined for each hemisphere, and the results were combined for each subject. Interrater reliability was assessed by reviewing region of interest placement of the two analysts. Thirty (93.8%) of 32 region of interest placements were within one adjacent pixel.
When the statistical mapping method, a more automated process, was applied, a Student's t test was performed on a pixel-by-pixel basis within a region of interest encompassing Brodmann's areas 17 and 18 of the visual cortex in each hemisphere (
7,
8). Only pixels reaching a predetermined significance threshold (t>3.33, p<0.001) were considered significantly activated. The percent activation in the significant pixels represents another measure of the amplitude of activation.
Statistical analysis. The main effect of sex on BOLD signal response and the percent activation in the significant pixels were assessed by one-way analysis of variance (ANOVA). The results from the mapping analysis were used to corroborate the primary measure of interest, BOLD signal response, which was then used in further analyses. Such analyses included independently testing the effect of hemisphere on BOLD signal response by using a repeated measures ANOVA model with hemispheric BOLD signal response as the repeated measurement. All results are presented as means and standard deviations. All p values reported reflect two-sided tests.
RESULTS
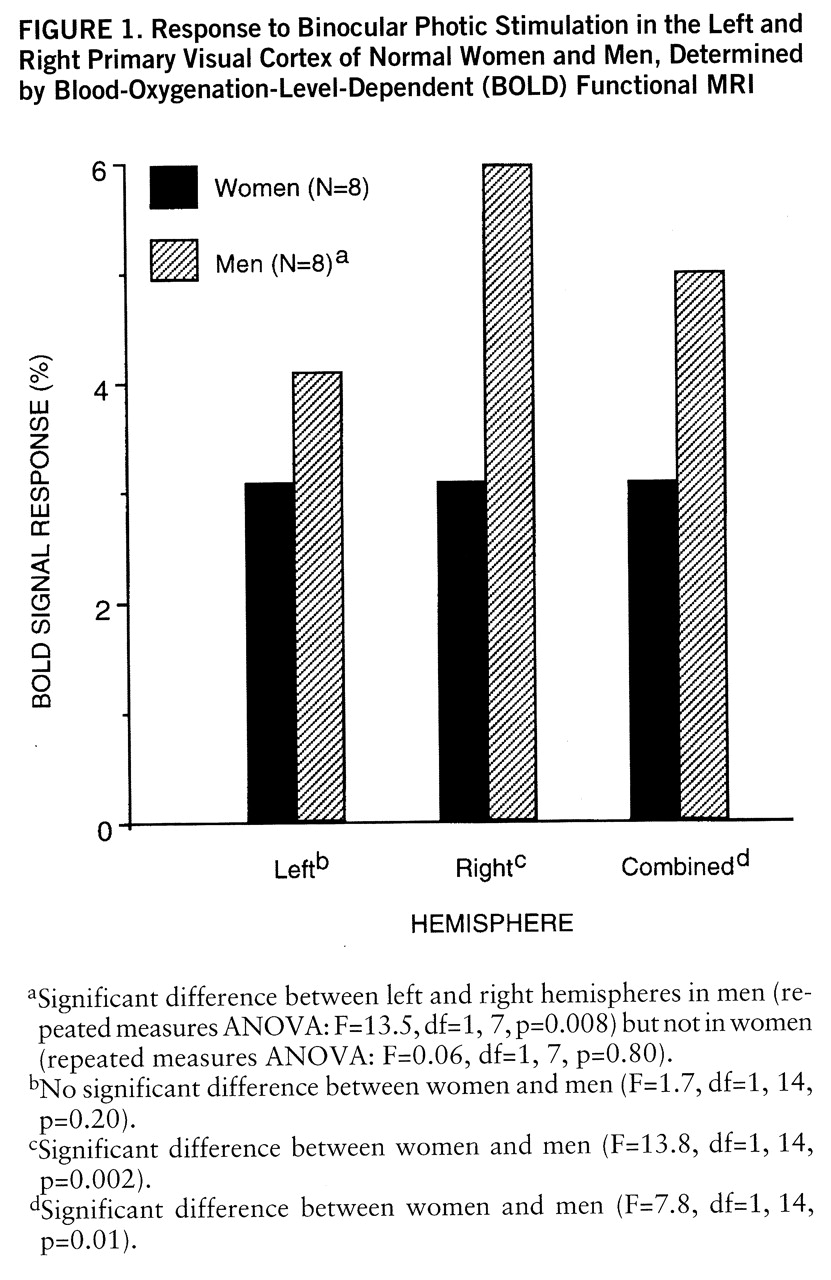
We found that women had a significantly lower mean BOLD signal response than men (mean=3.1%, SD=1.4%, versus mean=5.0%, SD=1.4%) (F=7.8, df=1,14, p=0.01), a difference of 38% (
figure 1). The semiautomated mapping method corroborated this finding: the women had a significantly lower mean percent activation in the significant pixels than men (mean=1.9%, SD=0.5%, versus mean=2.5%, SD=0.7%) (F=4.7, df=1,14, p<0.05), a difference of 24%.
In addition to the main effect of sex, the influence of hemisphere was evaluated with the sex-by-hemisphere interaction term by using a repeated measures ANOVA model. We found this interaction to be highly significant (F=9.8, df=1,14, p=0.007): women were more symmetrical than men, and much of the sex difference in BOLD signal response was attributable to the asymmetrical BOLD signal response in men (
figure 1).
DISCUSSION
We have demonstrated that women have a smaller BOLD functional MRI response to photic stimulation than a group of similar, age-matched men. This finding may reflect sex-based anatomical differences in visual cortex, differences in visual processing, differences in regional oxygen utilization with activation, differences in the vascular response to activation, or differences in baseline physiological measures related to BOLD contrast, such as hemoglobin level. However, the most likely explanation is multifactorial.
Although there is little evidence of sex-based anatomical differences in the visual systems, there is evidence of differences in visual physiology. Women have a larger amplitude in pattern-reversal evoked potentials and a higher amplitude in resting EEG as well as more pronounced EEG activity in response to photic stimulation than men (
9). On the other hand, although less directly relevant to our study, which used a primary visual stimulation, studies of visuospatial processing have consistently favored men (
10), and others have shown a greater visual system hemispheric specialization in men (
11).
The direction of our findings may appear to be in contradiction to these physiological data, as well as to the body of literature suggesting higher global and regional cerebral glucose metabolism and blood flow in women (
1–
5), but several points must be considered. The relationship between EEG activity and blood flow remains uncertain, and the previous imaging studies evaluated either resting conditions or cognitive activations, raising the issue of performance. We attempted to avoid the potential confound of cognitive task performance by assessing a simple primary sensory stimulus that requires little input on the part of the subject.
Perhaps more importantly, BOLD functional MRI evaluates brain activation in a manner that is fundamentally different from radiotracer methods. Although sensitive to changes in blood oxygenation states that accompany activation and thus related to cerebral blood flow and metabolism, it does not directly measure either parameter. It is sensitive to a complex interplay of flow, volume, and oxygenation, so that results are particularly dependent on baseline conditions. One baseline condition to which BOLD functional MRI theoretically may be particularly sensitive is hemoglobin concentration, since it is the iron within heme that provides the contrast in MR signal intensity. Although we did not measure hemoglobin levels in these subjects, young women have, on average, lower hemoglobin levels than men and, thus, perhaps less of a substrate for a BOLD functional MRI signal response to a focal activation.
Further studies must be performed to clarify the effect of this and other baseline factors that may systematically differ by sex. However, given our findings of a significantly greater hemispheric lateralization of the BOLD signal response in men, consistent with previous work suggesting greater visual system hemispheric specialization in men (
11), global factors are unlikely to be the sole determinant of our findings. Therefore, in addition to any possible functional significance, these results demonstrate that the effect of sex should be considered in the design and interpretation of functional MRI studies, although further study is needed to clarify the mechanisms underlying these data.