Shortly after the tricyclic antidepressants were introduced, a concern developed with respect to their cardiovascular toxicity, which was prompted by the observation that overdoses of tricyclic antidepressants resulted in death from cardiac complications (
1). Subsequently, systematic studies of the tricyclic antidepressants in patients with and without cardiac disease documented a number of cardiovascular effects, most notably 1) increase in heart rate, 2) orthostatic hypotension, 3) slowing of intraventricular cardiac conduction, and 4) type IA antiarrhythmic activity (
2). Until recently, the proven efficacy of the tricyclic antidepressants combined with detailed information on their adverse cardiovascular effects led to the conclusion that in most cases, tricyclic antidepressants could be given to depressed patients with cardiac disease. However, data from the Cardiac Arrhythmia Suppression Trial (
3,
4) indicated that this conclusion needed to be revised. The Cardiac Arrhythmia Suppression Trial was designed to demonstrate that suppression of ventricular premature depolarizations in the post-myocardial-infarction patient would reduce mortality. However, contrary to expectations, there was a substantial increase in the mortality rate among patients treated with a type IC antiarrhythmic agent (encainide or flecainide) compared with placebo-treated patients (
3), and a similar question was raised about moricizine, a type IA agent (
4). Consistent with the results of Cardiac Arrhythmia Suppression Trials I and II are studies that suggest an increased risk of mortality among patients with atrial fibrillation treated with quinidine, also a drug with type IA action, compared with placebo-treated patients (
5,
6). Although the mechanism for this increased mortality has not been definitely established, evidence suggests that an interaction between class I antiarrhythmics and ischemic myocardium leads to the greater mortality rate (
7–
9). Since tricyclic antidepressants have a class I antiarrhythmic action similar to that of quinidine or moricizine, in the absence of data to the contrary, it is reasonable and prudent to assume that tricyclic antidepressants also have similar risks (
10).
In addition to the reconsideration of the safety of tri~cyclic antidepressants in patients with ischemic heart disease, there is compelling new evidence that after a myocardial infarction, patients who are depressed have a higher mortality rate than comparable post-myocardial-infarction patients who are not depressed. In a study by Frasure-Smith et al. (
11), 222 patients were evaluated approximately 1 week after myocardial infarction, and 16% of this group met the DSM-III-R criteria for major depression. After control for established cardiac risk factors, the depressed patients had a 3.5 greater risk of dying a cardiac death during the subsequent 6 months than post-myocardial-infarction patients not diagnosed as depressed. Whether treatment of this depressive episode would reduce the associated increase in cardiac mortality has not been demonstrated.
However, before one can consider studying this question, it needs to be established that there is a safe and efficacious antidepressant treatment for post-myocardial-infarction depressed patients. The tricyclic antidepressants are an effective treatment for depression in patients with heart disease, but unfortunately their safety is suspect in patients with recent myocardial infarction. A natural question is whether the selective serotonin reuptake inhibitors (SSRIs) are a better alternative to the tricyclic antidepressants for depressed patients with cardiac disease. To date, the available data on the cardiovascular effects of the SSRIs have been collected only as addenda to outcome studies of medically healthy depressed patients. It is reported that the SSRIs slightly decrease heart rate, do not routinely slow intracardiac conduction, and do not affect supine or standing systolic or diastolic blood pressure (
12–
14).
This article reports the results of a study on the cardiovascular effects of an SSRI, fluoxetine, in the treatment of 27 depressed patients with preexisting cardiac disease: impaired left ventricular function, ventricular arrhythmias, and/or conduction disease. The cardiovascular effects of fluoxetine are then compared with the cardiovascular effects previously established for the tricyclic antidepressant nortriptyline.
METHOD
The patients included in this report were inpatients on a depression research unit who gave written informed consent to participate in a protocol studying the cardiovascular effects of antidepressant medication. They met the DSM-III-R criteria for major depressive disorder, unipolar subtype, and had a Hamilton Depression Rating Scale score of 16 or more. In addition, to be eligible for this study, at least one of the following conditions was required: 1) impaired left ventricular function defined as an ejection fraction ≤50% as determined by radionuclide angiography, 2) frequent ventricular arrhythmia defined as ≥10 ventricular premature depolarizations per hour as determined by the average of two consecutive 24-hour continuous ECG recordings, or 3) intraventricular conduction disease defined as a QRS interval ≥0.10 seconds as determined by 12-lead ECG recording.
Eligible patients who consented to participate in the research protocol were admitted to the hospital. If they were receiving a psychotropic medication, it was washed out for 2 weeks (6 weeks if the patient had been receiving any dose of fluoxetine), and their cardiac medication regimen was reviewed and stabilized by the supervising cardiologist. The patients then began a 2-week placebo period during which baseline cardiovascular data were obtained, including results of radionuclide angiography, two 24-hour continuous ECG recordings, 12-lead ECGs, and blood pressure measurements. Blood pressure was measured three times daily according to the following procedure: supine blood pressure was measured after 5 minutes of quiet rest, and then the patient stood for 1 minute and the blood pressure measurement was repeated. If at the end of the 2-week placebo period the Hamilton depression score was still 16 or more, the patient entered the medication treatment protocol.
A pilot study of fluoxetine was followed by an open-label protocol that randomly assigned patients to fluoxetine or nortriptyline treatment, with the subjects allocated three to one in favor of fluoxetine. The primary goal of this study was to establish the cardiovascular effects of fluoxetine in depressed patients with cardiac disease. A second goal was to compare the fluoxetine results to the effects of a tricyclic. Although a study with equal assignment to both fluoxetine and nortriptyline treatment would allow for a direct comparison of the SSRI with a tricyclic antidepressant, such a study would require a very large number of patients. Recruitment of such a patient group is difficult and the treatment expensive, and since there are already ample data on the cardiovascular effects of tricyclics in depressed patients with cardiac disease, it was decided that unbalanced random assignment would allow us to learn more about the SSRIs in a shorter period of time. Furthermore, if the randomly selected nortriptyline patients in this unbalanced design were comparable to patients in previous nortriptyline studies, it would allow for the comparison of the current fluoxetine group with a “historical” group of nortriptyline patients.
The dosing schedule of fluoxetine for both the pilot study and the random assignment study was 20 mg/day for the first 2 weeks and 40 mg/day during week 3. If the patient had not recovered by the end of week 3, the dose was increased to 60 mg/day for 4 more weeks. Although this dose escalation is more rapid than is recommended, a study objective was to detect adverse cardiac effects that might occur only at the highest dose of drug generally used in clinical practice.
The nortriptyline dose was calculated at 1 mg/kg of body weight; one-third of that dose was given on days 1 and 2, two-thirds was given on days 3 and 4, and the patient began the full dose on day 5. One week later the plasma level was measured, and the nortriptyline dose was adjusted, if necessary, to achieve a plasma level in the range of 50–150 ng/ml, which is established as the therapeutic “window” for this drug.
For the fluoxetine-treated patients, cardiac testing, including radionuclide angiography, 24-hour continuous ECG recordings, and ECG, was done at baseline and repeated at the end of week 2 of treatment (fluoxetine dose=20 mg) and of week 7 (fluoxetine dose=60 mg). For all nortriptyline-treated patients, cardiac tests were done at baseline and repeated at week 3 when nortriptyline was at a therapeutic plasma level. Blood pressure continued to be measured three times daily in all patients throughout medication treatment. To obtain mean supine and standing blood pressure measurements for the predrug and medication periods, the blood pressure readings were averaged for the 2 drug-free weeks and for weeks 2 and 7 in the fluoxetine patients and week 3 in the nortriptyline patients. Drop in orthostatic blood pressure was defined as the supine systolic blood pressure minus the standing systolic blood pressure. For the purposes of this study, a patient was considered to have orthostatic hypotension that required discontinuation of drug treatment if 1) an orthostatic drop of 50 mm Hg or more was recorded on three separate measurements, or 2) the patient was unable to maintain a standing position because of symptoms associated with an orthostatic drop of 30 mm Hg or more. Patients were also discontinued from the study if the baseline QRS interval increased by more than 50% during medication or if ventricular ectopic activity during medication met the proarrhythmia criteria as defined by the Cardiac Arrhythmia Pilot Study (
15).
To determine whether fluoxetine caused significant cardiovascular effects, paired t tests were used to compare mean baseline measures with means at week 2 and week 7 for supine and standing systolic and diastolic blood pressure, orthostatic drop, and heart rate (as determined by 24-hour continuous ECG recordings) in all patients; QRS and QTc intervals in patients with baseline QRS intervals ≥0.10 seconds; ejection fraction in patients with a baseline ejection fraction ≤50%; and ventricular arrhythmia in patients with ≥10 ventricular premature depolarizations per hour at baseline.
To compare the cardiovascular effects of fluoxetine with those of nortriptyline, we used two comparison groups. The first group was composed of patients who were treated with fluoxetine in either the pilot study or the current random assignment study. The second group was composed of patients who were treated with nortriptyline in the current study and patients who were treated with nortriptyline in our previous cardiovascular safety studies (
16,
17). Over the past 12 years, our group has conducted a series of studies on the cardiovascular effects of antidepressant medications. The psychiatric and cardiac inclusion and exclusion criteria for these studies have remained the same. To demonstrate that the patients included in the nortriptyline historical comparison group were comparable to the patients treated with fluoxetine in the current protocol, the two groups were compared with respect to age, sex distribution, and baseline cardiac status by unpaired t tests. The cardiovascular parameters at baseline were compared with week 2 measurements for fluoxetine and week 3 measurements for nortriptyline by paired t tests. Analysis of variance (ANOVA) was used to analyze the change scores for fluoxetine at week 2 compared with the change scores for nortriptyline at week 3 on the cardiovascular variables.
RESULTS
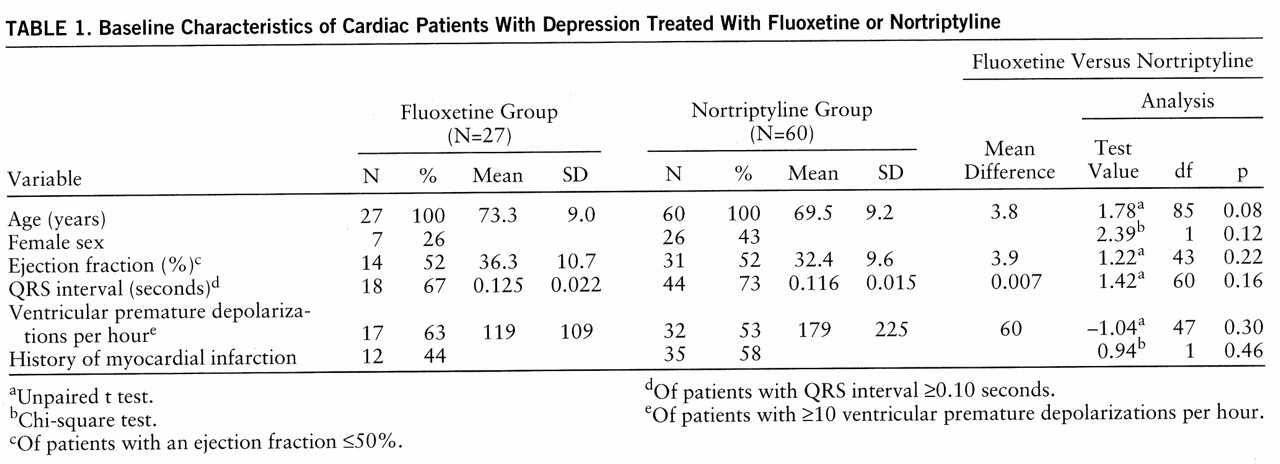
There were no significant differences between the fluoxetine and nortriptyline groups at baseline with respect to age, sex distribution, ejection fraction, QRS duration, and ventricular premature depolarizations per hour (
table 1) or cardiac medications. Twenty-seven patients (five from the pilot study and 22 from the random assignment study) met the inclusion and exclusion criteria, completed baseline testing, and began fluoxetine treatment. Baseline characteristics of the groups are shown in
table 1. At baseline 22% of the patients were taking no cardiac medication, 33% were taking digoxin, 25% a vasodilator, 33% a calcium channel blocker, 18% an ACE inhibitor, 18% a diuretic, 11% an antiarrhythmic, and 11% a β blocker.
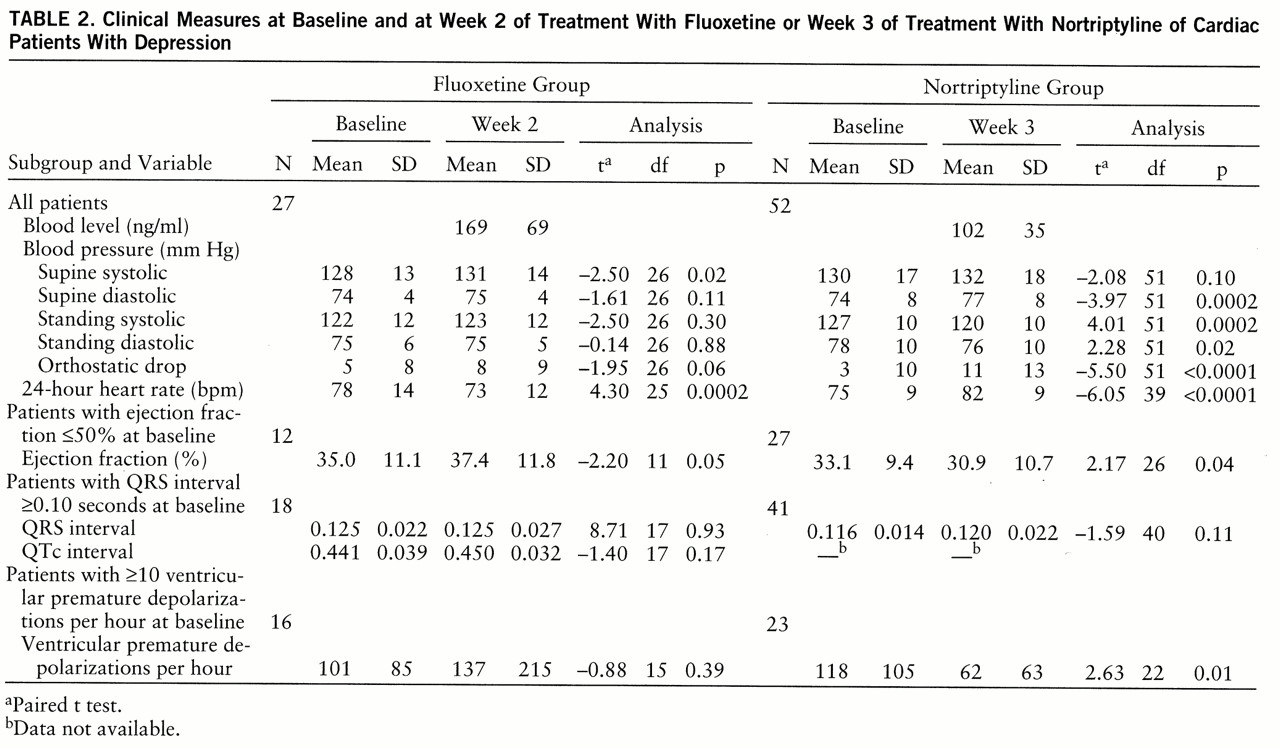
Values for clinical variables at baseline and at week 2 of fluoxetine treatment are presented in
table 2. Fluoxetine induced a 6% decrease in heart rate and a 2% increase in supine systolic pressure, both of which were statistically significant. In patients with a baseline ejection fraction ≤50%, fluoxetine induced a statistically significant 7% increase in the ejection fraction. In patients with a QRS interval ≥0.10 seconds at baseline, fluoxetine had no significant effect on either QRS or QTc intervals. Similarly, in patients with ≥10 ventricular premature depolarizations an hour at baseline, fluoxetine demonstrated no evidence of either a pro- or antiarrhythmic effect. There were no significant findings that emerged at week 7 that were not evident at week 2, despite the fact that the mean plasma level of fluoxetine plus norfluox~etine (the active metabolite) increased from 169 ng/ml (SD=69) at week 2 to 654 ng/ml (SD=287) at week 7.
There were 60 patients (seven from the random assignment study and 53 from the previous nortriptyline studies) in the nortriptyline comparison group. Baseline characteristics of these patients are shown in
table 1. At baseline 35% of the nortriptyline group were taking no cardiac medication, 40% were taking digoxin, 25% a diuretic, 20% a vasodilator, 15% an antiarrhythmic, 13% a calcium channel blocker, 8% a β blocker, and 7% an ACE inhibitor. Baseline clinical values and those at week 3 of nortriptyline treatment are presented in
table 2. Nortriptyline induced a statistically significant 4% increase in diastolic supine blood pressure, as well as a 5% decrease in standing systolic blood pressure and almost a threefold increase in orthostatic blood pressure drop. Nortriptyline also induced a statistically significant 9% increase in heart rate. In patients with a baseline ejection fraction ≤50%, there was a statistically significant 7% decrease in the ejection fraction. In patients with a baseline QRS interval ≥0.10 seconds there was no significant prolongation of the QRS interval, and in patients with ≥10 ventricular premature depolarizations an hour, there was a statistically significant 47% decrease in frequency.
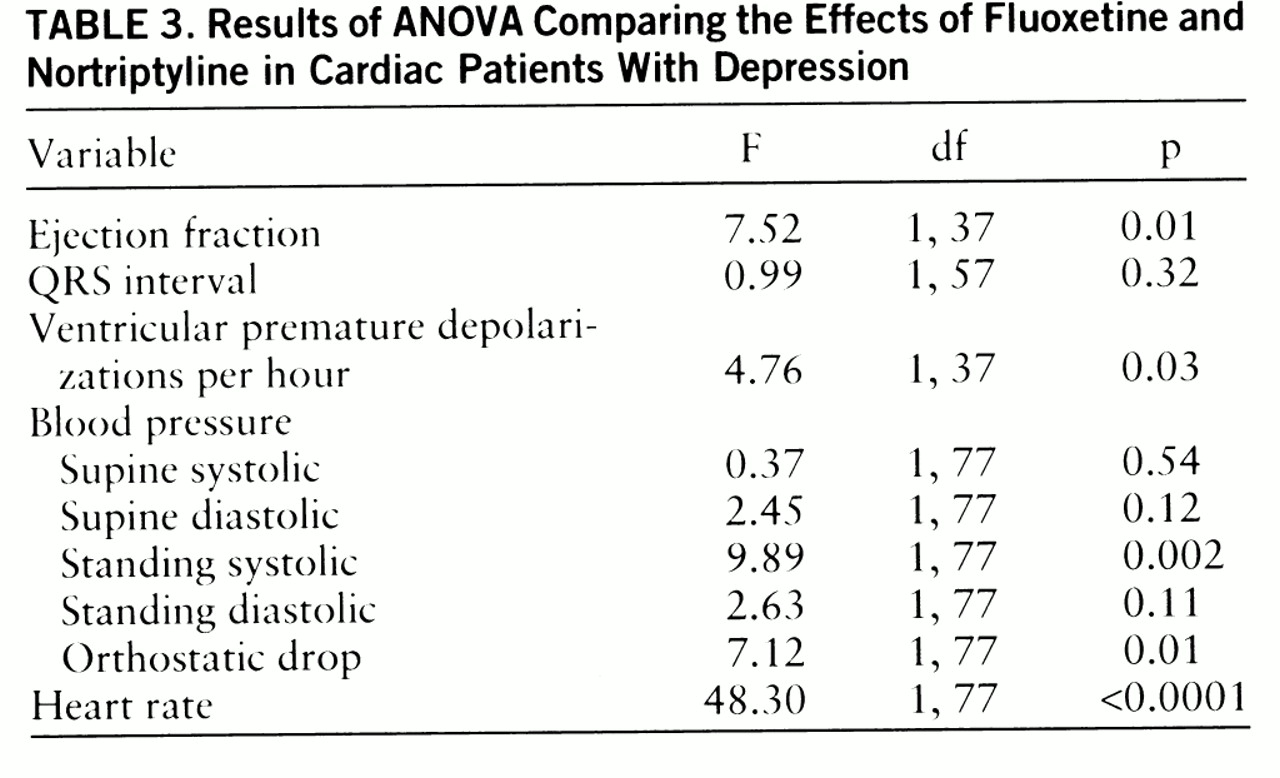
The effects of fluoxetine and nortriptyline on measures of cardiac function were compared by ANOVA (
table 3). The nortriptyline effect was significantly different from the fluoxetine effect with respect to heart rate, standing systolic blood pressure, orthostatic hypotension, ventricular premature depolarization frequency, and ejection fraction.
In both the fluoxetine group and the nortriptyline group there was a substantial dropout rate. Eight (30%) of the 27 fluoxetine-treated patients did not complete the medication trial; three patients left against medical advice, one patient developed temporal arteritis, one developed a syndrome of inappropriate antidiuretic hormone secretion that resolved after discontinuation of the drug, and two developed substantial agitation that resolved upon discontinuation of the drug. One patient with arrhythmia at baseline had increasing arrhythmia during the medication trial. After discontinuation of the medication, the degree and complexity of ventricular arrhythmia continued to increase for the next 4 weeks. No patient died during the fluoxetine treatment.
In the nortriptyline group, 15 (25%) of the 60 patients failed to complete the 3-week drug trial; four patients developed intolerable orthostatic hypotension, three patients had a proarrhythmic effect, two had acute myocardial infarction, two developed conduction complications (2:1 AV block), one developed severe, progressive congestive heart failure, and three had substantial anticholinergic symptoms. There were two deaths during the nortriptyline drug treatment.
Although the cause-and-effect relationship between nortriptyline or fluoxetine and the adverse cardiac events that occurred while patients were taking the medications may be strong in some cases and less clear in others, the most conservative approach to the data is to consider all cardiovascular events to be adverse drug events. Using this approach, we found that the patients taking nortriptyline had a 20% rate (N=12 of 60) of cardiac adverse events compared with a 4% rate (N=1 of 27) for those taking fluoxetine (p=0.06, Fisher's exact test).
DISCUSSION
The major finding of this study was that fluoxetine appeared to be a benign treatment for depressed patients with cardiac disease. Fluoxetine did not demonstrate the cardiovascular effects shown by nortriptyline. Nortriptyline has less orthostatic effect than the other tricyclic antidepressants and for this reason is considered to be the tricyclic of choice for elderly depressed patients (
17). Therefore, the use of a therapeutic plasma level of nortriptyline as the comparison treatment in studying fluoxetine is a rigorous standard. Fluoxetine lacked the cardiovascular effects well documented for nortriptyline and other tricyclics, specifically, increased heart rate, orthostatic hypotension, cardiac conduction abnormalities, and class I antiarrhythmic activity. With respect to left ventricular ejection fractions, nortriptyline caused a small but statistically significant negative effect, whereas fluoxetine had a small but statistically significant beneficial effect. A comparison of nortriptyline and fluoxetine showed approximately a 5% difference in ejection fraction, which was not only statistically significant but reached a level that is considered to be clinically significant. However, this finding with respect to left ventricular function must be considered with caution. Previous studies of tricyclic antidepressants, including nortriptyline, did not demonstrate a negative effect on left ventricular function (
16,
18–
20), and there is only a single case report of a documented decrease in ejection fraction induced by tricyclics (
21). Furthermore, this is the first study of fluoxetine in patients with left ventricular impairment, and it includes only 12 patients. Before we conclude that the SSRIs and tricyclics have a meaningfully different effect on ejection fraction, this result needs to be replicated.
One finding in this study that replicates the results of several previous studies is the effect of fluoxetine versus nortriptyline on heart rate. Nortriptyline increased and fluoxetine decreased heart rate; both findings have been previously reported for these drugs specifically, and there have been parallel results for other tricyclics and SSRIs (
13,
14,
16,
22,
23). In this study nortriptyline increased heart rate by 10% over baseline, and this effect may cause an increase in cardiac work. If so, this result, extended over the 6 months that successfully treated depressed patients would remain on a regimen of nortriptyline, could contribute to a potentially significant adverse effect.
The results of this study must be considered in the context of the methodological limitations of the design. First, the fluoxetine data were collected in an open treatment setting. However, the methods of data collection (e.g., radionuclide angiography, 24-hour continuous ECG recording, and automatic blood pressure recordings) are relatively resistant to bias, and the persons responsible for these measurements were not informed of the patients' medication status. Furthermore, the criteria for discontinuing medications because of orthostatic hypotension, conduction abnormalities, or proarrhythmia were defined in the protocol. Nonetheless, it may be that open treatment biased the results. Second, the nortriptyline and fluoxetine groups were not created by random assignment. Although the patients treated with nortriptyline and the patients treated with fluoxetine appear to be comparable with respect to age, sex, and type and severity of cardiac disease, the lack of random assignment increases the possibility that there were differences between the groups that may have influenced the cardiovascular medication effects and adverse events. However, this possibility is somewhat mitigated by the fact that the fluoxetine studies and the nortriptyline studies were done by the same research group, using the same methods, equipment, inclusion and exclusion criteria, and criteria for protocol discontinuation. Therefore, it seemed more informative and not unreasonable to use our own historical comparison group rather than simply compare the fluoxetine results with those reported in the literature for tricyclic antidepressants.
The findings of this study not withstanding, it is premature to consider fluoxetine a “safe” medication for patients with cardiovascular disease for many reasons, the most prominent of which is the relatively small number of patients studied (N=27). There still may be infrequently occurring but important adverse cardiovascular events that this study did not have the power to detect, and not all cardiac conditions were studied; for example, so far we have no information on the effect of fluoxetine in patients with sinus node disease nor data on patients in the immediate post-myocardial-infarction period. Furthermore, fluoxetine's effects on the cytochrome P450 system and the multiple medications that are a necessary part of a cardiac patient's treatment raise concern about possible drug-drug interactions. This study was not designed to test for such interactions, and although none was observed, we believe no conclusions should be drawn from these limited data. Finally, the length of this study, 7 weeks, precludes consideration of whether important cardiovascular effects may develop after prolonged treatment with fluoxetine, which is often necessary for the treatment of affective disorders.
When deciding on a treatment plan for a depressed patient with heart disease, the clinician must consider the risk-benefit ratio of any intervention. With respect to the risk, fluoxetine, and perhaps the SSRIs as a group, are substantially safer than nortriptyline and, undoubtedly, the tricyclics in general. However, there may be some types of depressive illness for which the tricyclics have better-established efficacy than do the SSRIs (
24). Therefore, the treatment of the depressed patient with heart disease remains a challenge for the clinician; decisions should be made on a case-by-case basis, taking into account the type and severity of the depression as well as the type and severity of the cardiovascular disease and the established cardiovascular effects of the various antidepressant medications.