METHOD
CSF was obtained from 18 patients who fulfilled the DSM-III-R diagnosis of alcohol dependence; they comprised four women and 14 men and had a mean age of 37.5 years (SD=8.4, range=27–53). Their drinking was in the moderately severe range; they had a score on the CAGE (
21) of 3.1 (SD=1.0) and a score on the Michigan Alcoholism Screening Test (
22) of 40.9 (SD=9.7). They had been drinking for an average of 20.7 years (SD=9.1); the total amount of absolute ethanol consumed in the last 6 months was 24.7 kg (SD=17.4), and the lifetime amount was 417.7 kg (SD=259.3). None of the patients had a history of withdrawal or other seizures. Other than ethanol and nicotine dependence (11 subjects), the study group did not have any history of substance abuse in the 6 months before admission to the National Institute on Alcohol Abuse and Alcoholism (NIAAA). None of the patients had any lifetime use of intravenous drugs or crack cocaine.
There was no evidence of cirrhosis or decompensated liver disease in the subjects. Mildly elevated levels of liver function indices were shown in laboratory tests performed upon admission to the study. The mean for alanine aminotransferase was 50.4 U/liter (normal range=6–41), the mean for aspartate aminotransferase was 57.3 U/liter (normal=9–34), and the mean for γ-glutamyltransferase was 114.6 U/liter (normal=14–84); all were consistent with recent drinking. Despite the level and duration of drinking in this group, there was no overt laboratory or clinical evidence of hypomagnesemia, myopathy, anemia, severe malnutrition, or infection, indices of common complications of chronic alcoholism.
Two CSF samples were acquired by lumbar puncture at 8.6 days (SD=1.9) and 33.0 days (SD=2.2) of abstinence from ethanol. Patients were admitted to the NIAAA research ward and enrolled in the study 1 week before the first lumbar puncture. During the period of study, the patients did not receive any psychotropic medication, including benzodiazepines. For the first 24 hours after admission, scores on the Clinical Institute Withdrawal Assessment Alcohol Scale (
23) were monitored hourly. Any patients with sustained scores of 12 or above were treated with benzodiazepines and excluded from the study. Although this procedure excluded patients with severe alcohol withdrawal, we wished to avoid the introduction of a factor (benzodiazepine medication) with a high likelihood of influencing the CSF measures. We did not attempt to perform lumbar puncture during the acute withdrawal phase. Rather, our study was designed to investigate subacute alcohol withdrawal and the intermediate postwithdrawal period.
Comparison CSF was obtained from age-matched subjects, three women and 15 men with a mean age of 38.3 years (SD=9.0, range=27–54). Subjects who had a history of neurological disorders, psychiatric disorders, or major medical illness were excluded. After complete description of the study to the subjects, written informed consent was obtained. The study was approved by the NIAAA intramural program institutional review board.
Both the alcoholic subjects and healthy comparison subjects were placed on a low-monoamine diet at least 3 days before the lumbar puncture. All subjects fasted after midnight and were kept at strict bed rest the morning of lumbar puncture. Lumbar puncture was performed with the subjects in the left lateral decubitus position between 9:00 and 10:00 a.m. CSF was collected by 1-ml fractions. The same fractions of the CSF were subjected to the assays described in the following sections.
Amino Acids and Peptides
Amino acids were measured by
o-phthalaldehyde precolumn derivatization coupled with a reverse-phase C-18 column for high-performance liquid chromatography separation and fluorescent detection (
24). The absolute concentrations of the amino acids were determined by computer analysis of peak height with internal and external standards.
N-Acetylaspartylglutamate (NAAG) and
N-acetylaspartate (NAA) were measured by anion-exchange high-performance liquid chromatography separation and ultraviolet detection at a wavelength of 214 nm (
25). Their concentrations were determined by peak height calculation as well.
Superoxide Dismutase
Total superoxide dismutase activity in CSF was analyzed by a modification of a spectrophotometric method (
26). The reduction of cytochrome
c was used to detect and measure superoxide dismutase activity. The reduction of acetylated cytochrome
c by ·O
2– is accompanied by a change in absorbance at 550 nm. Aliquots of CSF were added to 20 mM bicarbonate buffer, containing 10 µM sodium azide, 10 µM cytochrome
c, 100 µM xanthine, and 1 mM EDTA. The reduction of acetylated cytochrome
c was initiated by the addition of xanthine oxidase.
Lipid Hydroperoxides
The levels of lipid hydroperoxides were measured according to an adaptation of the ferrous oxidation/xylenol orange method described by Puttfarcken et al. (
27) by using 10 µl of CSF.
Protein Carbonyl
The method for protein carbonyl assay was adapted from the one described by Vevine et al. (
28), and we used 90 µl of CSF.
Statistics
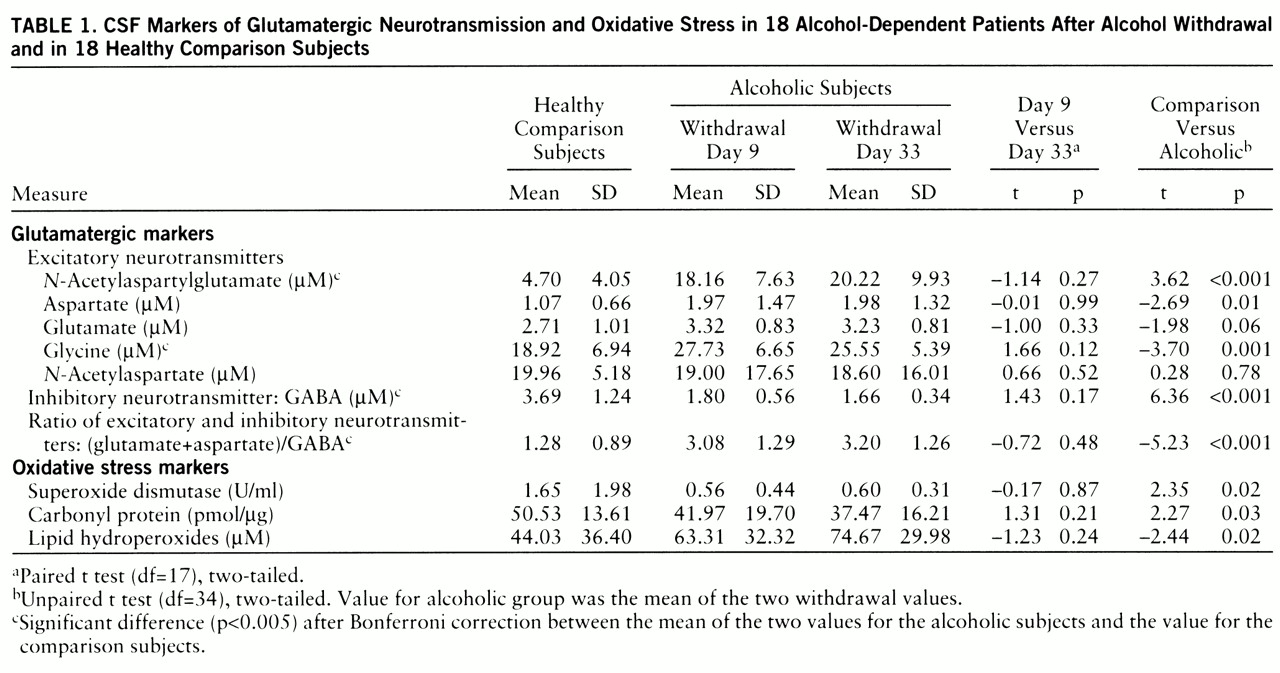
We first performed paired t tests (two-tailed) of the differences between days 9 and 33 and discovered no significant change in the neuro~chemical measures between these two time points (
table 1). We next derived means for days 9 and 33. The means for these two time points were compared to the values of the normal comparison subjects by unpaired t tests (two-tailed). Because of the multiple analyses, Bonferroni correction was applied (
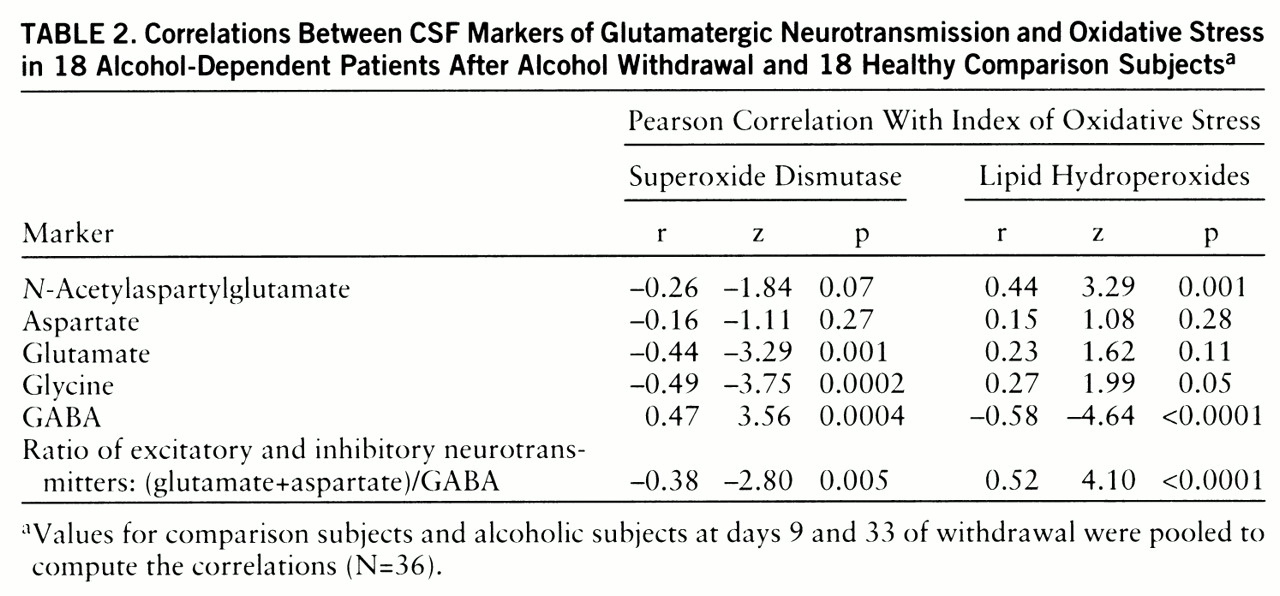
table 1). To estimate their contributions to the variance, we computed Pearson coefficients for the correlations between the markers of oxidative stress and the glutamatergic markers.
DISCUSSION
The chronically alcohol-dependent patients in this study who underwent mild to moderate alcohol withdrawal (scores less than 12 on the Clinical Institute Withdrawal Assessment Alcohol Scale) had higher concentrations of CSF indices of excitatory neurotransmission, NAAG and glycine, than did healthy comparison subjects (
table 1,
figure 1). We considered glycine to be a marker of excitatory neurotransmission because of its role as a coagonist of the NMDA receptor (
29), although it can also serve as an inhibitory neurotransmitter in the spinal cord (
30).
Since we do not have data from CSF obtained before alcohol withdrawal, we can not ascertain whether the differences that we observed were state dependent (due to withdrawal) or trait related (due to alcoholism). The values of glutamate, glycine, and NAAG observed in this cohort of comparison subjects were similar to those in comparison CSF from another study in this laboratory (
31), suggesting that the differences do not reflect a bias in the comparison samples. The patients' measurements during alcohol intoxication and after the patients presumably returned to their baselines after prolonged abstinence are not available. These present findings may either have preceded the alcoholism itself or be the consequence of long-term alcoholism, not directly associated with the state of alcohol withdrawal.
The relative contributions of inhibitory and excitatory pools of glycine in CSF are not clear. It is also unclear how the low-monoamine diet may have affected the neurochemical markers studied. However, it is conceivable that the stable environment and regular diet can decrease oxidative stress, and hence attenuate excitatory neurotransmission (
32), in alcoholics, who often encounter dietary deficiencies and various medical complications. Therefore, our findings of greater than normal excitatory neurotransmission after alcohol withdrawal are more likely due to either the withdrawal state per se or the preexisting condition of chronic alcoholism, which persisted into the withdrawal state, than to the diet itself.
Symptoms of alcohol withdrawal can last long after cessation of alcohol use. In our patients the neurochemical changes persisted up to 1 month of abstinence (
table 1). To the extent that the concentrations in the CSF represent synaptic events, the neurochemical differences may reflect intermediate- and long-term effects of alcohol withdrawal after moderately severe alcohol consumption. However, it will be important to study the neurochemical changes during acute withdrawal, which may reveal larger changes, since the NMDA neuro~transmission up-regulation is reported to be most robust during the first 48 hours of withdrawal in animals (
12). In vivo magnetic resonance spectroscopy can also help to determine the extent of enhanced excitatory neurotransmission during acute withdrawal. Alternatively, it is plausible that patients who experience symptoms of alcohol withdrawal are vulnerable to withdrawal because of their enhanced excitatory neurotransmission at baseline. Further studies of at-risk and long-term abstinent populations need to address this question directly. If indeed the enhanced excitatory neurotransmission is a trait marker, it may help to identify a group at high risk for alcoholism. In either case, our findings support the proposal that the pathophysiological basis for the symptoms of alcohol withdrawal and alcohol-induced neurotoxicity may be mediated by enhanced excitatory neurotransmission.
In addition to high indices of excitatory neurotransmission, we also observed a low CSF GABA concentration during alcohol withdrawal. It is well established that GABAergic neurotransmission is also an important component in the neurobiological effect of alcohol because of its activation of GABA-gated chloride channels (
9). The combined effect of the inferred increased excitatory neurotransmission and decreased GABAergic neurotransmission would lead to a substantial amplification of the overall excitatory neurotransmission, as indicated by the ratio (aspartate+glutamate)/GABA (
table 1). The only previous study of CSF GABA concentrations among alcoholic patients from this research program did not demonstrate a change of GABA concentration after cessation of alcohol use (
33). This apparent discrepancy may be due to the different duration of abstinence in the subjects, which was longer in the previous study. In animal studies on the effect of extended exposure to alcohol on GABAergic function, a reduced coupling between the GABA recognition site on the GABA
A receptor and the chloride channel has been found (
34). Thus, alcohol withdrawal may be associated with a more serious impairment of inhibitory neurotransmission than indicated by CSF GABA concentrations. The coexistence of low inhibitory and high excitatory neurotransmission revealed in our CSF study needs to be investigated in neuroanatomical studies that address the neuronal circuitry of the glutamate and GABA systems. For example, the inhibitory feedback by cortical GABAergic interneurons to the glutamatergic neurons is one of the candidates to investigate.
Even though the patients experienced only mild to moderate alcohol withdrawal symptoms, the group that we studied had a long history of alcohol dependence. Chronic attenuation of glutamatergic neurotransmission by ethanol results in a compensatory up-regulation of NMDA receptors that persists after withdrawal (
10–
13,
35). Augmentation of glycine, a coagonist of NMDA receptors, can further enhance glutamatergic neurotransmission (
29). Glycine also acts as an inhibitory neurotransmitter at the strychnine-sensitive glycine receptor, which is concentrated primarily in the spinal cord (
30). Glycine sites on the NMDA receptor may not be saturated (
36). Thus, a higher glycine concentration may augment NMDA receptor function in cortical structures. Overall, the combined augmentation of the synaptic concentration of excitatory neurotransmitters during alcohol withdrawal, in concert with the up-regulation of the postsynaptic receptors, may contribute to the enhanced glutamatergic neurotransmission. Consistent with this inference, glutamate release is significantly enhanced in alcohol-preferring rats (
37). During ethanol withdrawal, dependent mice have 50%–70% more brain MK-801 binding sites, a marker for NMDA receptors, than nondependent control mice (
10). Ethanol-naive mice prone to withdrawal seizures have also been found to have more MK-801 binding sites in the hippocampus (
38). In addition, NMDA exacerbates, while MK-801 and other NMDA receptor antagonists attenuate, the occurrence and severity of seizures associated with withdrawal of ethanol after chronic intake (
2,
15).
We also found high CSF NAAG levels after ethanol withdrawal (
table 1). Physiological studies (
39–
43) have demonstrated that NAAG blocks the action of endogenous glutamate. However, NAAG is catabolized to glutamate and NAA by the specific high-affinity membrane-bound surface peptidase
N-acetylated-α-linked acidic dipeptidase (NAALADase) (
44). Through the action of NAALADase, NAAG may serve as a precursor to extrasynaptic glutamate. Furthermore, NAAG is an agonist at the metabotropic receptor mGLUR-3, which inhibits glutamate release (
45). In addition to the glutamatergic neurons, NAAG is also colocalized in subpopulations of monoaminergic and cholinergic neurons (
46). Thus, aminergic activation during alcohol withdrawal may contribute to the increased release of NAAG in the CSF. Whether the elevated levels of NAAG represent a compensatory protective mechanism against glutamatergic damage through its inhibition of NMDA receptors and glutamate release or a source of increased extrasynaptic glutamate remains unresolved.
We observed higher than normal lipid peroxide levels and low superoxide dismutase activity in the CSF of alcohol-withdrawn patients (
table 1). The differences, however, did not reach statistical significance after the Bonferroni correction. Bonferroni correction is applied to variables independent of each other. The neurochemical variables we measured are not independent of each other. Not only are oxidative stress markers related to each other (low superoxide dismutase activity can lead to high levels of lipid hydroperoxide and protein carbonyl), but glutamatergic markers are also related to each other. NAAG is the precursor of NAA, glutamate, and aspartate (
47). Glutamate and aspartate are regulated together in most excitotoxic conditions (
31). GABA is a product of glutamate through the action of glutamate decarboxylase. More than that, oxidative stress and glutamatergic neurotransmission are mutually enhancing (
20,
32). To balance between type 1 and type 2 errors, the physiological meaning of the changes in superoxide dismutase activity and lipid peroxide level need to be discussed.
There are many potential mechanisms by which ethanol can generate free radicals, leading to lipid peroxidation. Ethanol itself can induce a dose-dependent increase in lipid peroxidation in brain homogenates. Lipid peroxidation is related to the metabolism of ethanol and acetaldehyde by secondary pathways that are known to generate oxygen-related free radicals (
48). Also, formation of long-lived hydroxyethyl free radicals from ethanol may result in membrane damage due to an increase in lipid peroxidation (
49). In addition to lipid peroxidation, ethanol-fed rats display increases in the production of thiobarbituric acid-reactive material, the generation of H
2O
2, and OH·-like species (
50,
51). Formation of free radicals from ethanol is also mediated by a transition-metal- and cytochrome-P450-derived superoxide-dependent oxidizing species (
52). Ethanol-induced cytochrome P450 IIE1 is constitutively expressed in the central nervous system. The induction of P450 IIE1 may lead to increased generation of reactive oxygen species and hydroxyethyl radicals, contributing to oxidative stress in the brains of chronic alcoholics (
53,
54). Nevertheless, the mechanism and physiological implications of the trend toward lower protein carbonyl during alcohol withdrawal (
table 1) remain to be explored.
Superoxide dismutase is an enzyme critical in the detoxification of superoxide (
55). Generation of free radicals by ethanol can be prevented by superoxide dismutase (
56), suggesting that superoxide is the primary source for other oxyradicals resulting from ethanol administration. Our study revealed a long-lasting reduction in superoxide dismutase activity in CSF after alcohol withdrawal. When superoxide dismutase activity is decreased, neurons are more vulnerable to oxyradical injury, and this is consistent with the high levels of lipid hydroperoxides that we observed (r=–0.30, p=0.03). Parallel with our findings, reduced activity of superoxide dismutase has been found in pigs chronically fed ethanol (
57). In addition to the attenuation of superoxide dismutase activity, the antioxidants glutathione and vitamin E were also shown to be decreased by ethanol exposure, which can further weaken the defense against oxidative stress (
58,
59).
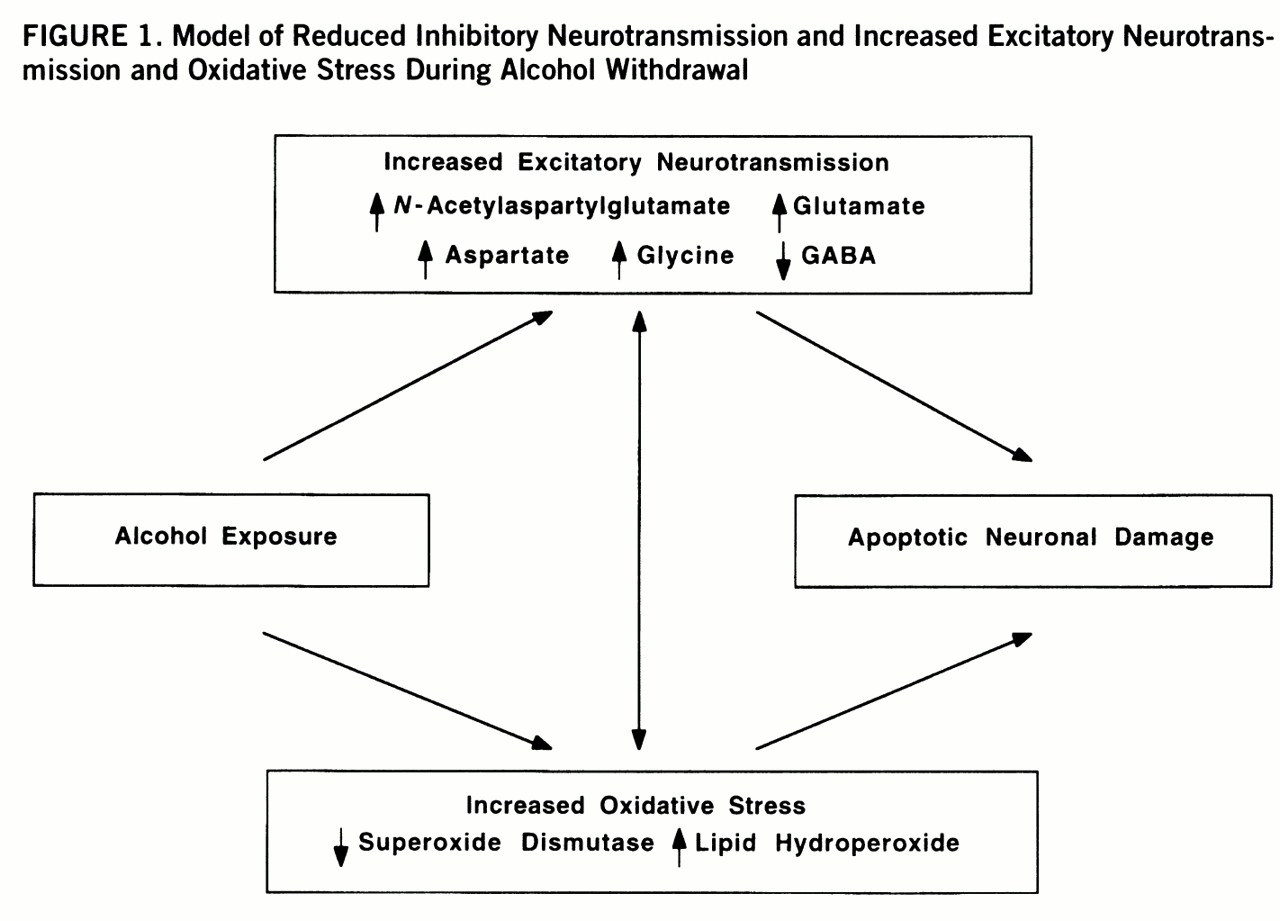
The negative correlations between CSF levels of excitatory neurotransmitters and superoxide dismutase activity and the positive correlations with lipid hydroperoxide levels suggest an important relationship between enhanced excitatory neurotransmission and oxidative damage after ethanol withdrawal (
figure 1). The activation of glutamate ionotropic receptors has long been known to cause selective neuronal degeneration (
60). Evidence indicates that the delayed form of glutamate-induced neuronal degeneration is mediated by reactive oxygen species (reviewed by Coyle and Puttfarcken [20]). Oxyradicals, depending on their source, can damage cellular proteins, membranes, and DNA, eventually causing cell death. In the case of alcohol withdrawal, increased lipid hydroperoxides may reflect free-radical-induced membrane damage. At the same time, formation of free radicals causes the release of glutamate (
32) and inhibits the sodium-dependent high-affinity transport process for glutamate (
61), the primary means for removing glutamate from the extracellular space. Thus, a vicious circle of mutually reinforcing processes due to increased glutamatergic neurotransmission and oxidative stress may contribute to neuronal damage in chronic alcoholism. The temporal sequence of the excitatory insult and oxidative stress cannot, however, be determined from these correlative findings but requires additional preclinical studies.
In summary, our data support the hypothesis that augmentation of excitatory synaptic neurotransmission leads to oxidative stress, which in concert with the up-regulation of postsynaptic glutamate receptors, may contribute to the neurotoxicity resulting from long-term alcohol abuse and even from a chronically mild alcohol withdrawal. One possibility suggested by the present data is that the enhanced excitatory neurotransmission not only is present at the acute withdrawal phase but persists for at least 1 month after withdrawal. This persistent effect may be due to a self-perpetuating cycle of enhanced glutamatergic neurotransmission and increased oxidative stress (
figure 1). Alternatively, these persistent alterations in excitatory markers in the CSF may represent metabolic traits associated with alcoholism. Another possibility is that chronic alcohol exposure per se augments excitatory neurotransmission and oxidative stress. Although our study could not delineate the time sequence of neuronal events induced by alcohol and did not resolve the trait versus state issue, the results further implicate glutamatergic dysfunction and oxyradicals in the pathophysiology of alcoholism and suggest potential treatments to attenuate neurotoxicity associated with alcohol withdrawal in dependent individuals.