Alzheimer's disease is the most common dementia of old age. Memory and attention are affected early, other cognitive domains—including language, executive, and visuospatial functions—become impaired later, and clinical deterioration progresses to death after about 10 years.
The brains of patients with Alzheimer's disease have regionally distributed intraneural fibrillary tangles composed of paired helical filaments, parenchymal amyloid-bearing plaques, neuron loss, and amyloid in blood vessels (
1–
3). Multiple neurotransmitter and metabolic abnormalities are consistent with widespread neural dysfunction and synaptic failure (
4–
9). Disease severity and cognitive impairment correlate positively with progressive synaptic dropout and dysfunction (
10,
11). Compared with association cortical areas, the visual, sensorimotor, and auditory primary cortical areas are relatively spared. Quantitative studies of visual cortex demonstrate 20- to 40-fold fewer neurofibrillary tangles in primary striate cortex than in visual association areas; neuritic plaque density, however, is comparable in the striate and association areas (
2).
In PET activation studies, stimulus complexity and severity of Alzheimer's disease independently influence regional CBF responses. In an earlier study (
20), we presented an alternating pattern-flash stimulus to a group of patients with Alzheimer's disease who had scores on the Mini-Mental State (
21) ranging from 0 to 28. Despite normal regional CBF values at rest and at low levels of stimulation (low flash frequency), at high stimulation levels the striate cortex in the Alzheimer's disease group had significantly lower CBF values than those in healthy comparison subjects. In other visual activation studies, visual association areas in mildly demented patients had CBF responses similar to those of healthy subjects at modest activation levels (
22,
23), whereas the CBF responses of moderately to severely demented patients in the same areas at the same modest activation were significantly smaller than those of healthy subjects (
23).
METHOD
Subjects
The consent forms were approved by the institutional review board. All subjects or their legal guardians gave written consent for participation in a protocol to evaluate regional CBF responses to a temporally graded visual stimulus. Subjects were recruited without regard for race, gender, or religion through advertisements in newspapers and on bulletin boards in local community buildings, from physician referrals, from other protocols in our laboratory, and from social service agencies. The patients with Alzheimer's disease met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (
26). Twenty-one patients were compared with 19 healthy subjects. The Alzheimer's disease group was divided into two subgroups on the basis of scores on the Mini-Mental State (
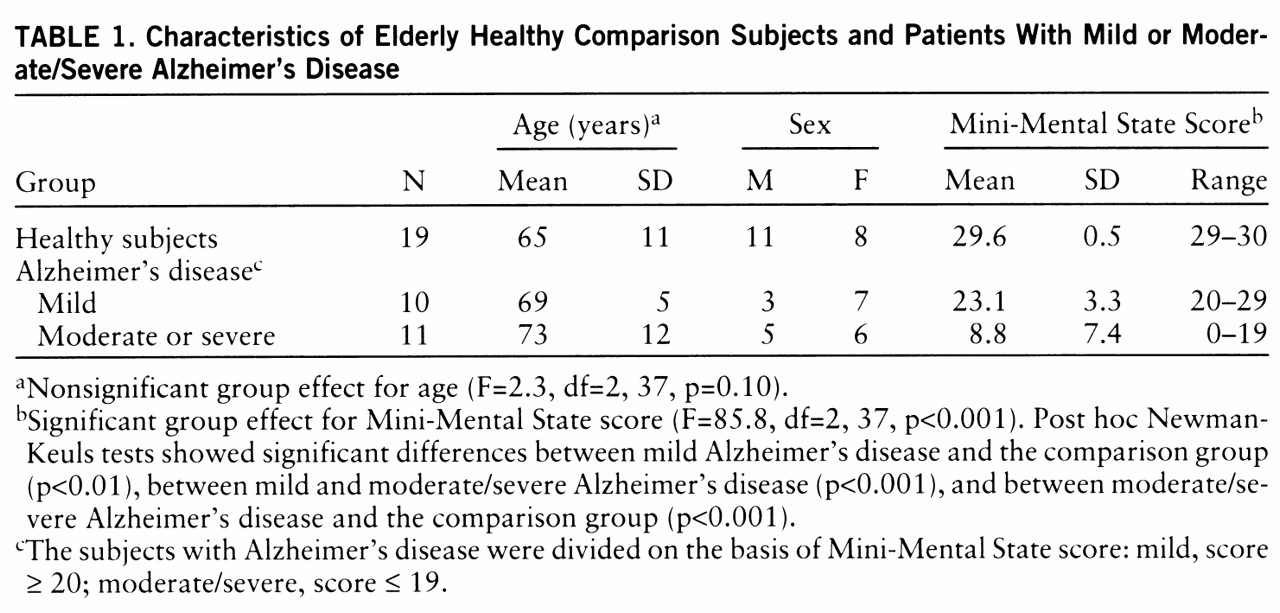
21): a mildly demented group that had scores of 20 or higher (N=10) and a combined moderately/severely demented group that had scores of 19 or lower (N=11). Demographic characteristics of the three groups are presented in
table 1. Age and sex were not statistically different among the groups.
No subject had a major medical disorder other than Alzheimer's disease, as determined by history, physical examination, chest X-ray, ECG, magnetic resonance imaging, and laboratory tests (complete blood count; measurement of sedimentation rate, electrolytes, glucose, blood urea nitrogen, creatinine, liver-associated enzymes, cholesterol, triglyc~erides, antinuclear antibodies, serum B12, and folate; thyroid function, VDRL, and HIV tests; and urinalysis). No subject had a visual abnormality as assessed by history and examination (ophthalmoscopy, tests of pupillary function and extraocular movement, acuity charts, and field integrity determined by confrontation response).
Pattern-Flash Stimulus
Each subject was scanned five times during a PET scan session. During each scan, a flashing stimulus was administered alternately to the two eyes at a single frequency by a pair of goggles (model S10VS B, Grass Instrument Co., Quincy, Mass.) taped to a thermoplastic face mask. Five scans for each subject were obtained at frequencies of 0, 1, 4, 7, and 14 Hz, administered in random order and counterbalanced across subjects.
Stimulus characteristics, CBF responses, and subjective percepts for the healthy subjects have been presented elsewhere in detail (
27). Briefly, the stimulus was delivered by goggles that contained a 6×5 rectilinear grid (1.52 cm by 1.27 cm) of 30 monochromatic red-light-emitting diodes, mean peak 655 nm, embedded into each eyepiece. The grids subtended an arc of illumination of about 50° and were flashed alternately into the left and right eyes, and the number of flashes presented to one eye per second determined the flash frequency in hertz. Flash frequency was varied by changing the duration of the interval between flashes. Flash duration was fixed at 5 msec. The luminous intensity of each flash remained constant at 3.6 mcd; therefore, as flash frequency increased, so did luminous intensity per unit time. The subjects were told to watch the flashing lights, no specific fixation point was required, and the test-retest reliability of the CBF responses in all brain regions was very high (
27).
The subjects, when able, described the subjective perception of the alternating pattern-flash stimulus as follows. At 0 Hz it appeared dark. From 1 through 14 Hz the image brightness steadily increased, reflecting increases in luminance. At 1 Hz the grids were seen as formless, dull red rectangles. As the flash frequency increased, the individual light-emitting diodes (LEDs) became visible and the LED pattern gradually emerged. That is, the form became more complex as the flash frequency increased. Many shades of orange and yellow, in addition to bright red, were reported at high frequencies, whereas at low frequencies the only color reported was dull red. Thus, as the flash frequency increased, image brightness (luminance) increased, form complexity increased, and multiple colors were perceived. At 1 Hz the dull red rectangle was seen alternately by the left and right visual fields and was reported to “oscillate” or move. This apparent motion occurred because the LED grids were not centered over each fovea. At higher frequencies the rate of the alternation of flashes between the left and right fields was too rapid for apparent motion and the two rectangles were perceived as a single larger flickering rectangle.
PET Scan
Regional CBF was measured by using [
15]H
2O with a positron emission tomograph (model PC2048-15B, Scanditronix, Uppsala, Sweden) that has reconstructed transverse and axial resolutions of 6.5 mm. Head movement was minimized during and between scans with a thermoplastic mask molded to each subject's head and attached to the scanner bed. A transmission scan was obtained during rotation of a
68Ge/
68Ga source around the subject's head to correct for photon attenuation by skull and cerebral tissue. Eight subjects from each group received 30 mCi of [
15]H
2O intravenously as a bolus; the rest received 40 mCi. During the 4 minutes after the [
15]H
2O was injected, data were collected simultaneously in 15 planes spaced 6.5 mm apart and parallel to the inferior orbitomeatal line. Sixteen images were obtained, 12 10-second images followed by four 30-second images. Automatic arterial blood sampling with an indwelling catheter was initiated at the time of injection and was continued throughout the scanning period. Data from the 16 images and the arterial time-activity curve were used to reconstruct the set of 15 slices composing each scan. Regional CBF was calculated by using a rapid least squares method (
28,
29).
Data Analysis
Values for CBF in all brain voxels across the five pattern-flash frequencies were compared in the patients with mild Alzheimer's disease, the patients with moderate or severe Alzheimer's disease, and the healthy comparison group. As we used arterial lines, we could compare group values of absolute mean global CBF in milliliters per 100 g per minute before data transformation. The group main effect for mean values was significant (F=31.3, df=2,197, p<0.001). According to post hoc Newman-Keuls tests, the patients with moderate or severe Alzheimer's disease had significantly lower global CBF than the patients with mild Alzheimer's disease (p<0.001), and the mildly demented group in turn had lower global CBF than the healthy comparison group (p<0.001). As a global difference reflects the disease process, a significant global CBF difference should not be ignored or minimized by normalization or scaling procedures before between-group regional CBF comparisons (
21,
30). Therefore, we performed the following stereotaxic normalization and data transformation.
By using statistical parametric mapping (
31), the images were registered, stereotaxically normalized into the space of the Talairach and Tournoux atlas (
32), and smoothed by 20 mm × 20 mm × 12 mm in the X, Y, and Z axes (X=left/right, Y=posterior/anterior, and Z=inferior/superior). To reduce effects of differences in global CBF between runs while maintaining the effect of the significant between-group global CBF difference, CBF at each voxel was normalized to the whole brain value (CBF at each voxel was divided by the global CBF of the scan from which it was derived) and then multiplied by the mean global CBF of the appropriate group (comparison, mild Alzheimer's disease, moderate/severe Alzheimer's disease). This procedure standardized values of regional CBF in milliliters per 100 g per minute (
21,
30).
The patient groups with mild and moderate/severe Alzheimer's disease were compared with the healthy comparison group at 0 Hz (resting scan) by using statistical parametric mapping (
31).
We previously demonstrated four major patterns of CBF response in different brain areas of healthy subjects as flash frequency increased (
27): 1) a biphasic rising then falling, 2) a linear rising, 3) a linear falling, and 4) a peak at 1 Hz in the middle temporal region V5/MT. Using appropriate contrasts in within-group statistical parametric mapping analyses (biphasic pattern, –2 1 2 1 –2; linearly increasing pattern, –2 –1 0 1 2; linearly decreasing pattern, 2 1 0 –1 –2), we identified areas displaying each of those regional CBF response patterns in the comparison group, the mildly demented patients, and the moderately or severely demented patients. In the analyses, voxels were thresholded at p<0.001, uncorrected for multiple comparisons, and then activation clusters that exceeded a p<0.05 two-tailed probability, corrected for multiple comparisons, were reported. To evaluate between-group differences, we performed a two-way repeated measures analysis of variance (ANOVA), with the variables group and frequency (repeated), at every voxel by means of a computer program written in C (formulae from a standard textbook [33]). In separate analyses, voxels having a significant group main effect or a significant group-by-frequency interaction were thresholded at p<0.01. Significant group main effects or group-by-frequency clusters of voxels that exceeded a p<0.05 two-tailed probability, corrected for multiple comparisons, were determined by using the NIH Functional Imaging Data Analysis Platform (written by Dr. J. Maisog) based on the analytic method of statistical parametric mapping (
31,
34). To illustrate the different CBF response curves in different brain regions, we analyzed local maxima from within the significant group main effect or group-by-frequency clusters. For each local maximum we present a graph of the CBF response that includes simple effects of group (Newman-Keuls test), and we also present the group main effect (two-way ANOVA), the group-by-frequency interaction (two-way ANOVA), and the main effect of frequency within each group (one-way ANOVA).
RESULTS
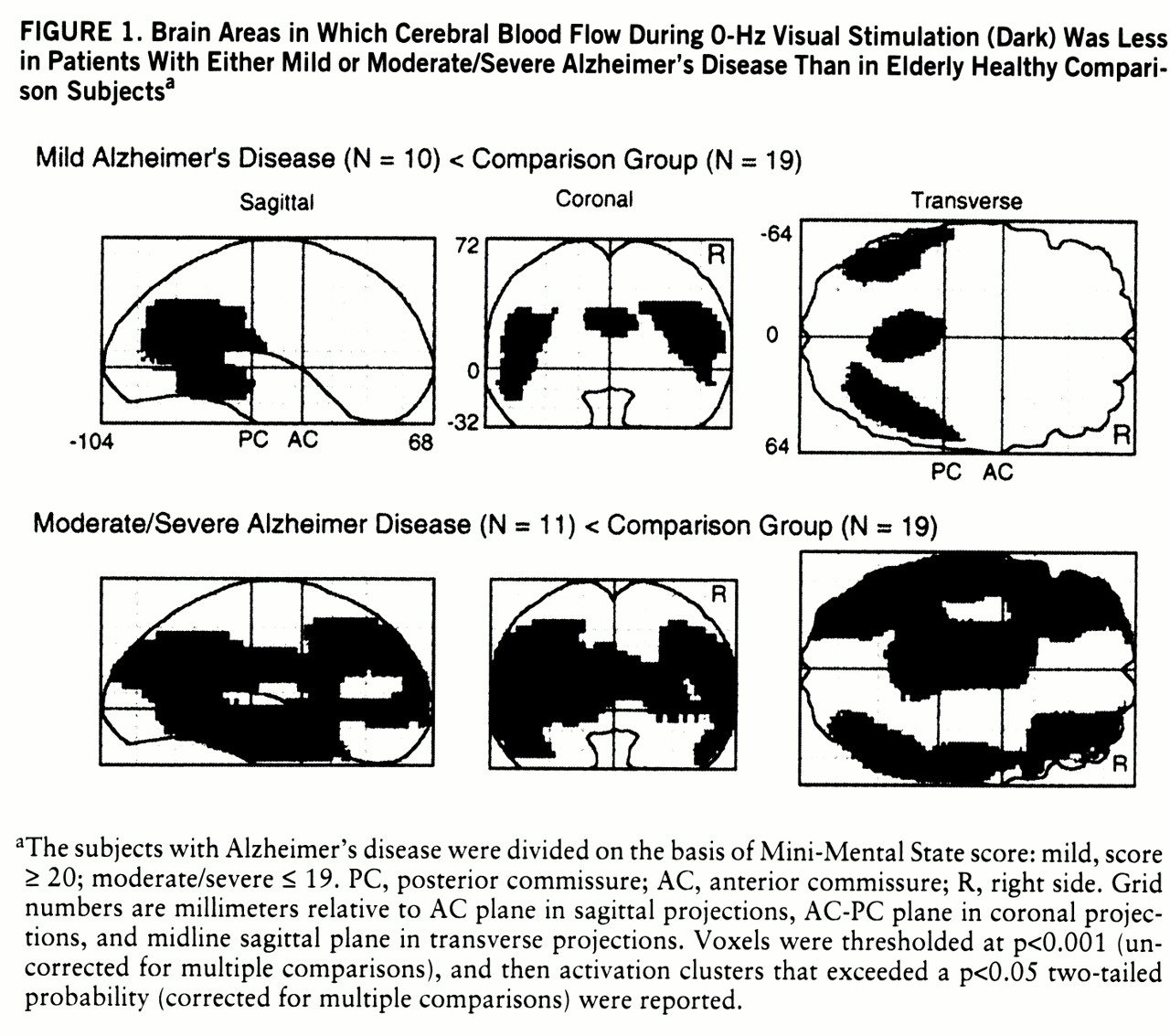
Figure 1 shows the brain areas in which regional CBF at rest (dark, 0 Hz) was significantly lower in the mildly demented and moderately/severely demented patients than in the comparison group.
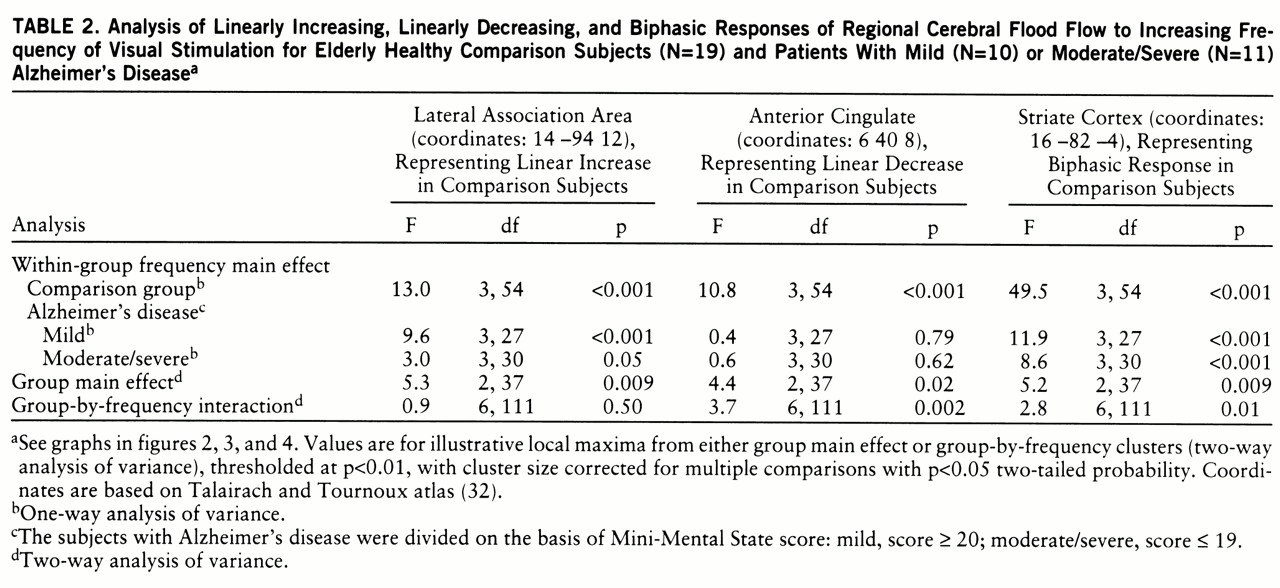
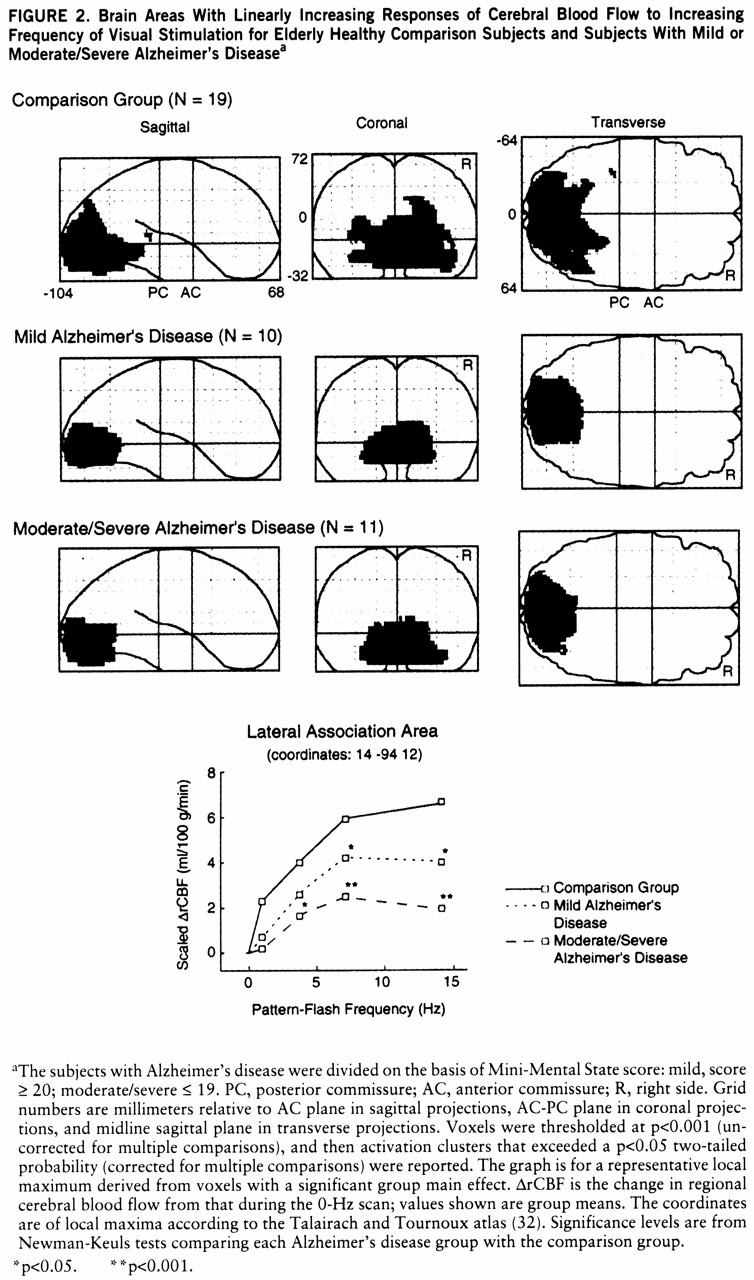
Figure 2 identifies the brain areas in which CBF showed linearly increasing change as flash frequency increased. From the striate cortex, significant responses in the comparison group extended inferiorly along the lingual and fusiform gyri (Brodmann areas 18 and 19) and laterally into the orbital gyrus, middle occipital gyrus, and cuneus (Brodmann areas 18 and 19). Similar regions, but with smaller volumes, were activated in both Alzheimer's disease groups. At a representative local maximum (lateral association area), derived from the voxels with a significant group main effect, the change in CBF increased significantly as flash frequency increased in all three groups (
figure 2 graph,
table 2 within-group frequency main effects). In relation to the comparison group, CBF change was significantly less in the patients with mild Alzheimer's disease at 7 and 14 Hz and in the patients with moderate/severe Alzheimer's disease at 4, 7, and 14 Hz (New~man-Keuls tests,
figure 2 and
table 3).
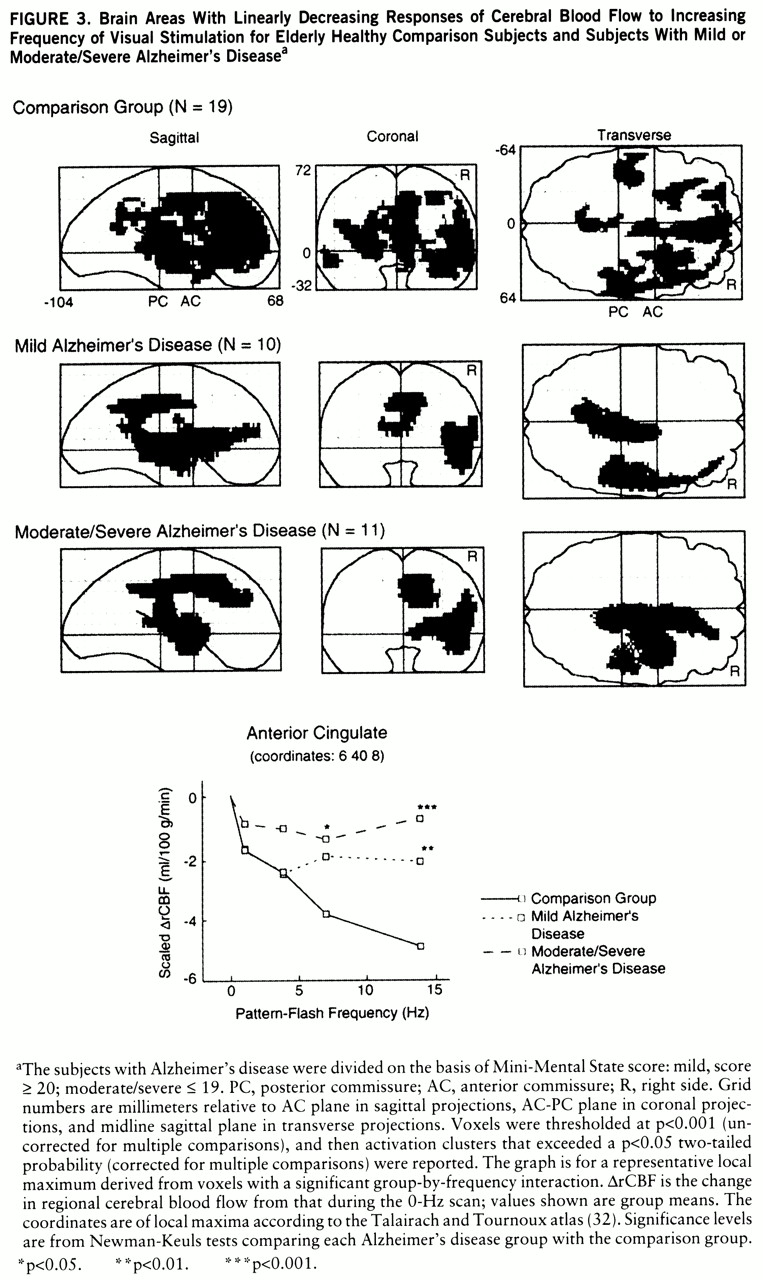
Figure 3 shows that many “anterior” brain areas in the comparison group had linearly decreasing values for CBF change as flash frequency increased (cingulate, medial and lateral frontal, superior temporal, parietal), with greater volumes of significant activation in the right than left hemisphere. For both Alzheimer's disease groups, the areas with significant linearly decreasing values of CBF change were almost exclusively in the right hemisphere (
figure 3). At a representative local maximum (anterior cingulate), derived from the voxels with a significant group-by-frequency interaction (
figure 3 graph,
table 2), regional CBF change significantly decreased with increasing pattern-flash frequency in the comparison group but not in the patients with mild or moderate/severe Alzheimer's disease (within-group frequency main effects). In relation to the comparison group, change in regional CBF was significantly less in the mildly demented patients at 14 Hz and in the moderately to severely demented patients at 7 and 14 Hz (
figure 3 graph).
We have published the extent of significant biphasic changes in CBF in striate cortex Brodmann area 17 in healthy subjects (
27).
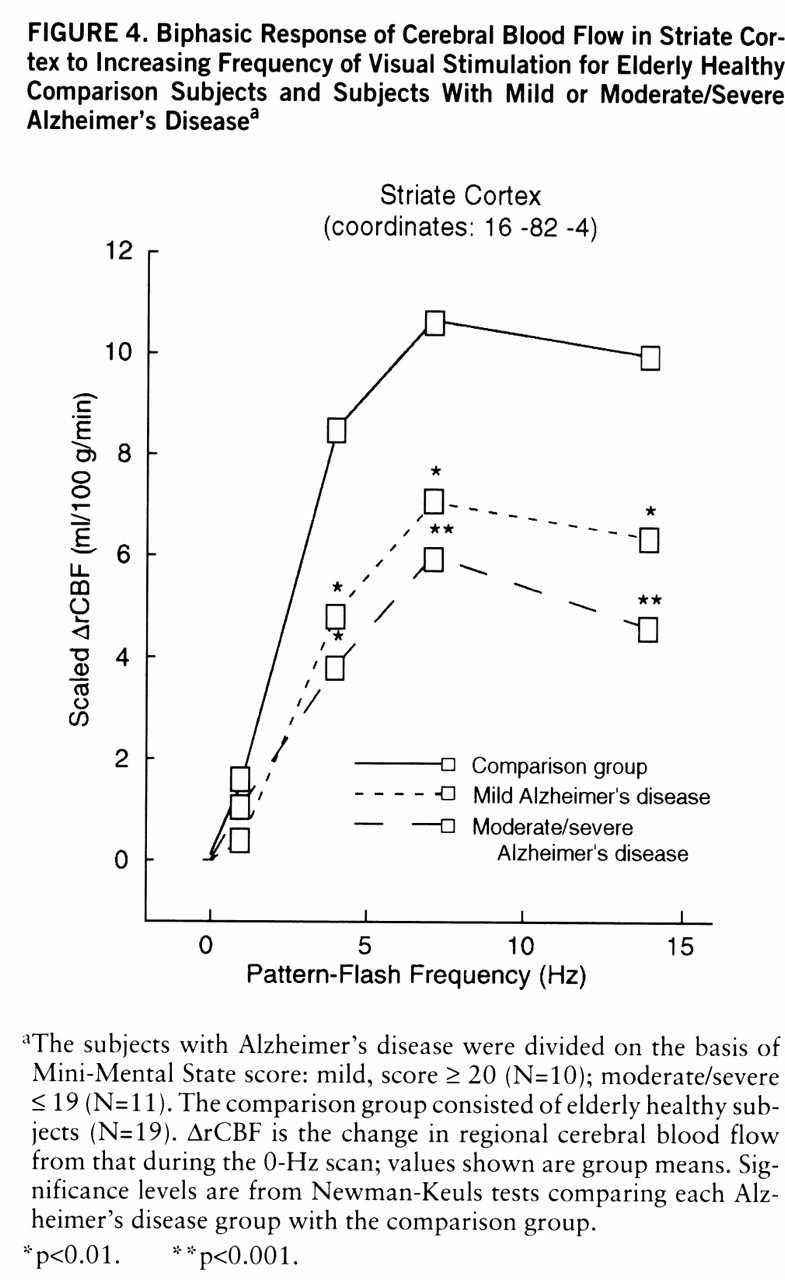
Figure 4 shows CBF change in the comparison, mild Alzheimer's disease, and moderate/severe Alzheimer's disease groups at a representative striate cortex local maximum derived from the voxels with a significant group main effect. The change in regional CBF was significant across all flash frequencies (within-group frequency main effects) and peaked at 7 Hz in all groups. In relation to the comparison group, both Alzheimer's disease groups had changes in regional CBF that were significantly smaller at 4, 7, and 14 Hz (
figure 4).
We previously reported a significant CBF response in middle temporal (V5/MT) areas at 1 Hz (frequency of apparent motion) in healthy subjects (
27) and demonstrated the absence of such activation in a group of patients with Alzheimer's disease of mixed severity (
21). In this study, the comparison group had a significant regional CBF response (mean=4.29 ml/100 g per minute, SD=5.8) at 1 Hz (t=2.4, df=18, p<0.05), whereas the responses of both Alzheimer's disease groups were nonsignificant (p>0.05). The value at 1 Hz of the comparison group was significantly greater than the value for the two Alzheimer's disease groups combined (t=2.4, df=38, p=0.02).
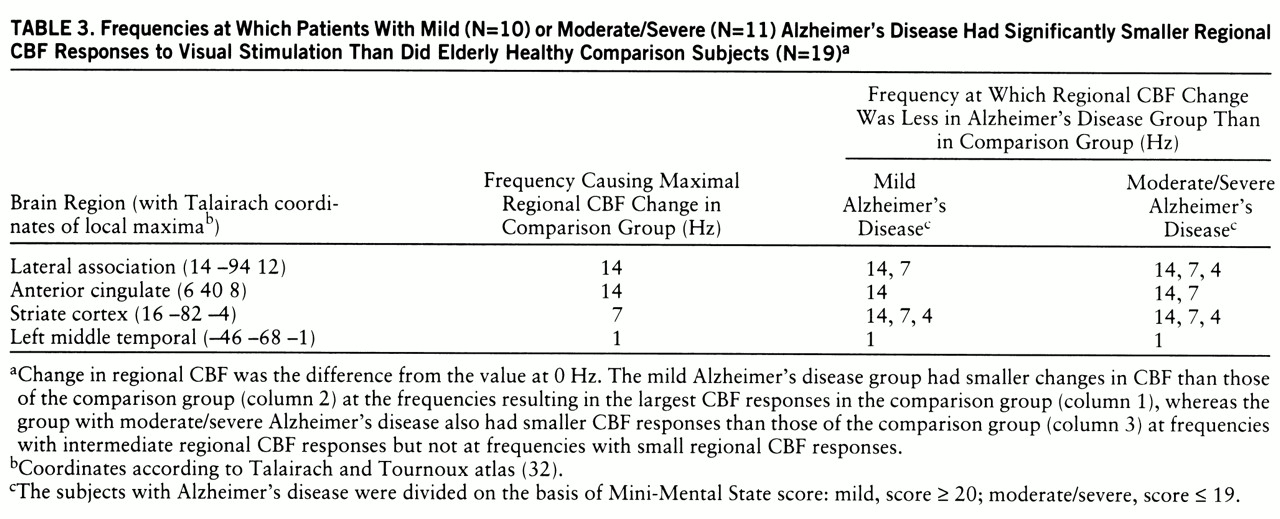
The first data column in
table 3 lists the frequencies that resulted in the largest changes in regional CBF in the comparison group (largest neural responses). Columns 2 and 3 list the frequencies at which the mild and moderate/severe Alzheimer's disease groups, respectively, had significantly smaller changes in regional CBF than the comparison group. For each brain area, the mild Alzheimer's disease group had a significantly smaller change in regional CBF at the frequency that resulted in the largest change for the comparison group, whereas the moderate/severe Alzheimer's disease group had a significantly smaller change at that frequency (largest change in comparison group) and also at frequencies that resulted in intermediate amounts of change in regional CBF in the comparison group, but not at frequencies that resulted in small changes.
DISCUSSION
The pattern-flash stimulus and its effect on brain function in healthy subjects have been described in detail elsewhere (
27). In summary, the subjects reported seeing a progressively more complex, bright, and colorful pattern as flash frequency increased, and at 1 Hz they reported seeing apparent motion. The CBF response to the pattern-flash stimulus in primary (striate) visual cortex was bimodal, increas~ing between 0 and 7 Hz and then falling at higher frequencies, reflecting the “activity-recovery cycle” (
35) of retinostriate pathways (
figure 4). CBF steadily increased from 0 through 14 Hz in Brodmann visual association areas 18 and 19 (lingual, fusiform, orbital, middle occipital gyrus, and cuneus), reflecting the increas~ing neural activity required to process increasingly complex images (
figure 2). Apparent motion caused CBF increases in the motion-processing area (V5/MT) at 1 Hz, and CBF decreased from 0 to 14 Hz in many anterior brain areas irrelevant for processing the visual stimulus (
figure 3).
In relation to the comparison group, the mildly demented patients with Alzheimer's disease had lower CBF values at rest (dark, 0 Hz) in temporoparietal regions (
figure 1), whereas the moderately to severely demented patients had significantly lower resting CBF extending bilaterally from the temporoparietal areas into the frontal lobes anteriorly and into the occipital lobes posteriorly (
figure 1). These results confirm findings from many previous studies of resting subjects conducted with PET and [
15]H
2O or [
18F]fluorodeoxy~glucose, and they are consistent with postmortem findings that the greatest density of Alzheimer's disease pathology is found in the temporoparietal lobes.
In the mildly demented group, almost all of the posterior and anterior brain areas activated by the pattern-flash stimulus (figures
2 and
3) had normal at-rest CBF values (
figure 1). In the group with moderate or severe Alzheimer's disease, the striate and visual extrastriate regions activated by the pattern-flash stimulus (figures
2 and
3) had normal at-rest CBF values (
figure 1). All areas activated by the pattern-flash stimulus in both Alzheimer's disease groups had significantly smaller changes in regional CBF than did the comparison group at one or more levels of activation (graphs in figures 2, 3, and 4). Therefore, in both the mildly and severely demented patients, abnormal function can be exposed in areas considered normal at rest by increasing brain stimulation (neural stress test).
However, the degree of stimulation required to expose functional abnormality was greater in the mildly demented patients than in the moderately or severely demented patients. The group with mild Alzheimer's disease had changes in regional CBF that were smaller than those of the comparison group at the frequency resulting in the largest change in regional CBF (large neural response) in the comparison group, whereas the moderately or severely demented group also had changes smaller than those of the comparison group at frequencies eliciting intermediate changes in regional CBF in the comparison group but not at frequencies with small changes (
table 3).
We previously demonstrated (
20) that the middle temporal cortex (V5/MT), responsible for processing motion, was activated at 1 Hz in healthy subjects but not in patients with Alzheimer's disease for whom results were averaged across all levels of dementia severity. In the present study, significant activation in the middle temporal gyrus (V5/MT) was again seen in the comparison group (mean change in regional CBF=4.29 ml/100 g per minute, SD=5.8) (t=2.4, df=18, p<0.05) but not in either the mildly or moderately/severely demented Alzheimer's disease group.
In the following sections we discuss the pathophysiological implications of these results at the levels of cellular function, neural subpopulations, and subjective information processing.
Increasing Disease Severity and Neural Failure
It has been amply demonstrated that changes in regional CBF are correlated with changes in brain metabolism (
36–
40). Furthermore, during neural activation the largest metabolic changes occur in synaptic elements, and thus change in regional CBF reflects primarily changes in synaptic activity (
41–
45).
Alzheimer's disease causes progressive accumulation of neurofibrillary tangles, amyloid plaques, neural dropout, loss of neurotransmitters, and reduction of synaptic binding sites. These pathological changes interact cumulatively to impair neural function and interneural communication (synaptic activity) and thus reduce PET regional CBF. We found smaller changes in regional CBF in the patients with moderate or severe Alzheimer's disease than in those with mild Alzheimer's disease; as disease severity increased, synaptic activity became progressively impaired. Our findings for mild Alzheimer's disease suggest that synaptic responsiveness is within normal limits for stimuli normally eliciting small or moderate increments in regional CBF but fails when stimuli should elicit large regional CBF responses. In moderate to severe Alzheimer's disease, synaptic activity is within normal limits only for stimuli that should elicit small changes in regional CBF and fails with stimuli requiring moderate or large regional CBF responses.
In Alzheimer's disease, dendritic sprouting and new synapse formation or modification occur early in the disease and may be compensatory responses to loss of neurons and synaptic elements (
46,
47). In a study of biopsied brain tissue from patients with Alzheimer's disease, DeKosky and Scheff (
11) found that the total synaptic surface area contact per unit volume was maintained by enlarging mean apposition length; as presynaptic density decreased, the remaining synapses increased in size. However, in brain tissue biopsied postmortem (more severe disease), the volumetric compensation for numerical loss of presynaptic elements was exceeded and both synapse number and appositional length were reduced. These authors hypothesized that this neural plasticity was an attempt to maintain synaptic functional integrity as Alzheimer's disease pathology progressed.
Our data suggest that whatever neuroplastic changes occur in the course of Alzheimer's disease to maintain functional integrity, they do so imperfectly in mild dementia, as our patients with mild Alzheimer's disease had impaired function when the neural response required was large. Our finding that functional failure occurred with progressively smaller neural responses as disease severity increased is consistent with the finding by DeKosky and Scheff (
11) that synaptic appositional length decreased in more severe disease.
Whether a neural stress test can expose abnormal neural function in preclinical subjects with minimal pathology is an important question for future research.
Differential Neural Subpopulation Vulnerability to Disease Pathology
In higher primates, including humans, most visual stimuli are transmitted by retinal cells along two separate neural pathways. Neurons in the magnocellular pathway have high contrast sensitivity, fast temporal resolution, and low spatial resolution and are color insensitive. Neurons in the parvocellular pathway have low contrast sensitivity, slow temporal resolution, and high spatial resolution and are color sensitive (
48–
51). Each of these separate pathways conducts visual information from the retina to the striate cortex via a single synapse in the lateral geniculate nucleus. From the striate cortex, magno- and parvocellular neurons project to all extrastriate visual association areas except the middle temporal area (V5/MT), which has an almost exclusively magnocellular input (
52,
53). Both magno- and parvocellular neurons can respond to low-frequency visual stimuli, but at high frequencies the magnocellular system is more efficient (
48,
49,
54).
In agreement with our earlier findings (
21), the CBF change in the striate cortex in each of the Alzheimer's disease groups was more impaired at high than low frequencies (
figure 4), suggesting that magnocellular function is more impaired than parvocellular function in the striate cortex in Alzheimer's disease. As the middle temporal area (V5/MT) has an almost exclusively magnocellular input, failure to activate middle temporal areas in both Alzheimer's disease groups at 1 Hz, the frequency at which apparent motion occurs, supports severe magnocellular dysfunction even early in Alzheimer's disease.
Passive Visual Information Processing
In healthy subjects the linear increase in neural function (change in regional CBF) in inferior and lateral association areas (
figure 2) in response to increasing flash frequency likely represents the neural biology underlying increasingly complex form, color, and luminance processing (
27). Smaller changes in regional CBF in the Alzheimer's disease groups, especially at higher frequencies, may reflect an inability to integrate percepts of luminance, color, and form as they become more complex. Change in regional CBF was significantly less for the mildly demented patients than for the healthy subjects at 7 and 14 Hz, suggesting that mildly demented patients have perceptual problems only at high levels of visual image complexity.
In the healthy subjects the anterior brain regions showed linearly decreasing changes in CBF as the flash frequency increased (
figure 3), reflecting progressive reduction in neural function in brain areas irrelevant for processing the passive visual stimulus (
27). The Alzheimer's disease groups were unable to decrease frontal neural activity to the same extent as the comparison group at high flash frequencies, that is, when the visual percepts were most complex. The neurophysiological mechanisms or circuits underlying these functional changes in healthy subjects are unknown.
Progressive abnormalities in high-level cognition, including memory, language, attention, and visuospatial and executive functions, characterize the dementia of Alzheimer's disease (
26) and are major foci of clinical research. However, the present study identifies functional abnormalities in Alzheimer's disease in striate and immediate extrastriate association areas (Brodmann areas 18 and 19), suggesting dysfunction in perceptual processes. As accurate perception facilitates accurate memory and visuospatial and executive functions, perceptual dysfunction likely contributes to the dementia of Alzheimer's disease. Successful drug treatment might be directed toward modulating perceptual networks in addition to those underlying the more traditional cognitive and attentional processes.
Conclusion
Using a passive parametric visual stimulus, we were able to systematically vary CBF response, an index of neural activity, in many brain regions. We exposed functional failure in brain regions that had normal function at rest and at modest levels of activation and that typically have modest (frontal and extrastriate cortex, Brodmann areas 18 and 19) or minimal (striate cortex) Alzheimer's disease pathology. Stimuli that resulted in large changes in regional CBF in healthy subjects were necessary to elicit functional failure in mild dementia, whereas stimuli resulting in intermediate as well as large changes in regional CBF elicited functional failure in moderate to severe dementia.
This stress test approach holds promise for screening preclinical at-risk subjects to identify functional abnormalities in the presence of minimal pathology and for evaluating drug therapies, for which changes in regional CBF responses to varying work loads might be used as objective physiological measures of drug efficacy.