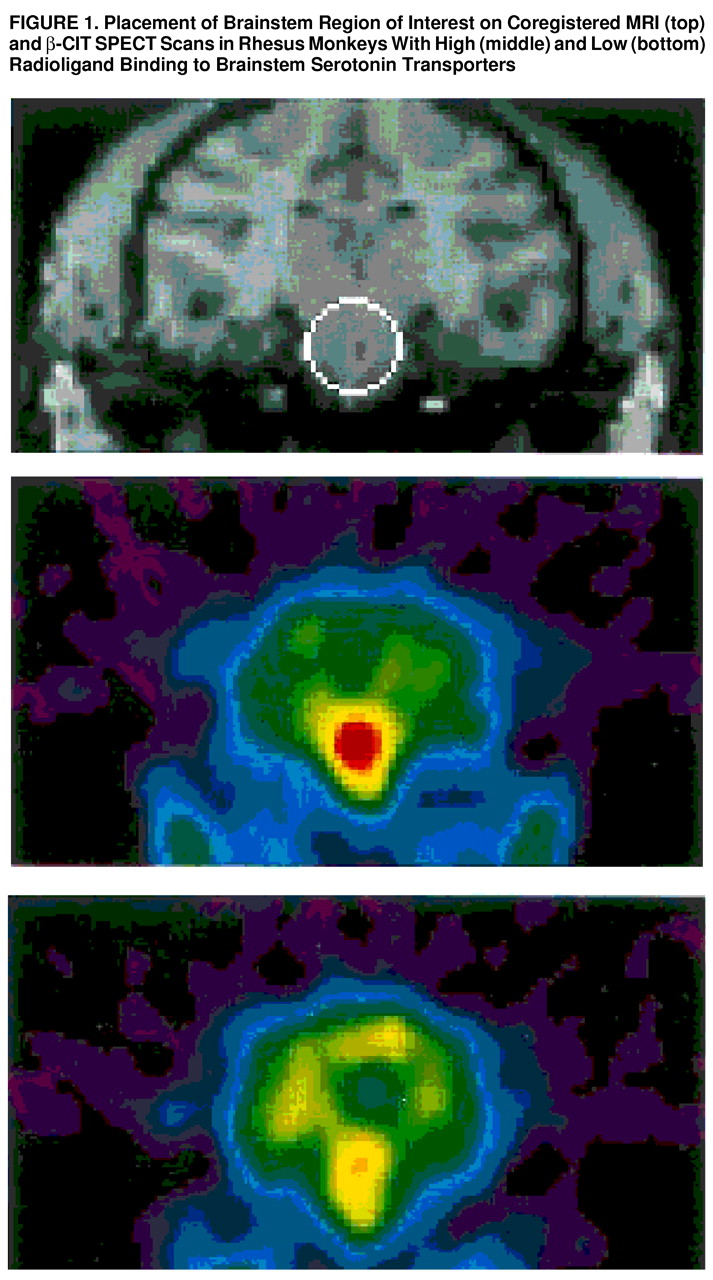
In this study of 11 rhesus monkeys, greater availability of the serotonin transporter as measured by [
I123]β-CIT binding was found to be significantly correlated with low levels of the serotonin metabolite 5-HIAA in CSF, less intoxication upon initial exposure to alcohol, and greater aggressiveness. Similar behavioral and biochemical patterns have been reported to predispose humans to the development of alcoholism (
11,
12). The correlation between [
I123]β-CIT binding to serotonin transporters in the brainstem and 5-HIAA in CSF may be due to a difference in the density of serotonin transporters or to a difference in the serotonin concentrations in the synapses, both of which can change the relative availability of serotonin uptake sites for the radioligand (
22,
31,
32). Either way, the implication of the findings is the same: animals with low CSF 5-HIAA concentrations and high transporter availability should have low serotonin concentrations in the synapse. Our findings suggest that interindividual differences in CSF 5-HIAA concentrations may have their genesis in altered serotonin transport and that CSF 5-HIAA concentrations may be related to the availability of serotonin transporters in the brainstem. Previous ex vivo findings in alcohol-preferring vervet monkeys support the hypothesis that low levels of monoamine metabolites in the CSF are associated with a higher density of monoamine transporters (
33).
Our findings are compatible with a role for dysregulated serotonin neurotransmission in the pathogenesis of excessive alcohol consumption. They indicate that CSF 5-HIAA concentrations are correlated with serotonin uptake in the brainstem, the site of the raphe nuclei and the central origin of cortical and subcortical serotonin projections (
29,
34). A dysfunction of serotonin transmission in the raphe system may be one of the environmentally and genetically influenced biological variables predisposing to excessive alcohol consumption (
35,
36). Among young rhesus monkeys, heritable influences account for more than 60% of the variance in 5-HIAA metabolite concentrations (
37). Among adult human and nonhuman primates, the influence of heritable variables on central serotonin turnover rates is less than 50% and environmental variables account for more than one-half of the variance (
9,
10,
38). In this study, all monkeys underwent social separation stress during infancy. A number of studies with nonhuman primates have shown that influences by adults, particularly maternal input, are critical to modulate the development of the central serotonin system (
27,
37,
39). In the absence of influence by adults, the development of serotonin function is impaired. When CSF 5-HIAA was obtained from 51 neonatal peer-only-reared and mother-reared monkeys on postnatal days 14, 30, 60, 90, 120, and 150, the peer-reared subjects exhibited lower CSF 5-HIAA concentrations than did the mother-reared subjects (
40). One study with a limited number of subjects suggested that the effect of early rearing experiences on CSF 5-HIAA concentration may disappear by adolescence (
18). However, in a larger study in which peer-reared and mother-reared subjects were longitudinally studied from infancy into adulthood, the peer-reared subjects exhibited lower CSF 5-HIAA concentrations than the mother-reared subjects in both infancy and adulthood (
9,
10). Similar findings have been observed by other investigators as well (
19). Our observation of a significant association between serotonin transporter availability and aggressiveness in adult nonhuman primates that had experienced parental separation during early development is interesting. It may be relevant to humans, because findings from a recent twin study (
41) also emphasized the importance of environmental factors in the pathogenesis of antisocial behavior among alcoholics.
In conclusion, our findings indicate that central serotonin dysfunction is associated with lower initial sensitivity to alcohol intoxication and with greater aggressiveness, two variables implicated in the pathogenesis of alcoholism. By characterizing the molecular neurobiology of addiction, the present in vivo imaging data may aid in the development of therapeutic strategies that target specific neurotransmission dysfunctions among alcoholics and other substance abusers.