Hypersensitivity to CO
2 inhalation is one of the most widely studied laboratory markers of panic disorder, and many studies have clearly demonstrated the ability of single- or double-vital capacity inhalations of gas mixtures with high concentrations of CO
2 (35%)
(1-
4) to induce acute anxiety/panic in patients with panic disorder. The nature of this association, however, is not yet clearly understood. Growing evidence suggests that hypersensitivity to 35% CO
2 might be a trait marker for panic disorder rather than a simple state marker. Anxiety reactivity to 35% CO
2 inhalations has been reported to be not significantly influenced by clinical characteristics of the disorder such as baseline anxiety, frequency of panic attacks, severity of agoraphobia, and duration of illness, as well as by age
(4) and personality
(5) dimensions. Recent studies have suggested that there might be a familial association between panic disorder and hypersensitivity to 35% CO
2. Rates of CO
2-induced panic attacks have been shown to be higher in healthy first-degree relatives of panic patients than in healthy control subjects
(6) or in healthy first-degree relatives of patients with mood disorders
(7), and morbidity risk for panic disorder in families of panic patients who are hypersensitive to 35% CO
2 was higher than that for families of panic patients with normal sensitivity to CO
2 (8).
Given these data, it can be speculated that CO
2 hyperreactivity might be the expression of an underlying familial vulnerability to panic directly related to its pathogenetic mechanisms. A relevant role of genetic factors in panic disorder is clearly supported by the literature. Many studies have reported the existence of a strong familial loading for panic disorder
(9,
10), and twin studies have reported a higher concordance for panic disorder in monozygotic than in dizygotic twins
(11-
14), supporting the genetic nature of this vulnerability. Given that the etiology of panic disorder is related to genetic factors, if there is a pathogenetic link between panic disorder and CO
2 hypersensitivity, it can be hypothesized that in 35% CO
2-induced panic attacks, genetic factors might also play a relevant role. Twin studies may clarify the relative contributions of genetic and nongenetic factors.
METHOD
Subjects
One hundred thirty-two same-sex pairs of twins, randomly taken from the Register-Office List of Twins covering all twin birth in Milan from 1957 to 1974, were personally contacted by telephone and were asked “to participate in a twin study dealing with the role of genetic factors in the pathogenetic mechanisms underlying anxiety disorders.” Fifty-five pairs (42%) accepted, but 10 were excluded because of contraindications related to the challenge (three for significant respiratory disorders, two for significant cardiac/circulatory disorders or hypertension, two for family history of cerebral aneurysm, one for epilepsy, and two for significant risk of pregnancy).
Exclusion criteria were significant cardiac/circulatory and respiratory disorders, personal or family history of cerebral aneurysm, significant hypertension (systolic blood pressure >180 mm Hg, diastolic >100 mm Hg), pregnancy, or epilepsy, all determined by direct physical examination and careful collection of medical histories.
At the time of the challenge, it was required that none of the subjects had taken any psychotropic medications during the last 2 weeks. They were asked to refrain from alcohol consumption for at least 36 hours, beverages containing xanthine for at least 8 hours, and food or smoking for at least 2 hours before the test.
The sample included 25 pairs of dizygotic and 20 pairs of monozygotic twins. Venous blood samples, anticoagulated with EDTA, were drawn from all probands. Genomic DNA was extracted from thawed blood by salting out with saturated NaCl solution. Determination of the twins’ zygosity was made by direct administration of a questionnaire that has been reported to predict zygosity correctly in 95% of cases, when compared with results obtained by blood analysis of genetic markers
(15). For each twin we also analyzed a hypervariable single locus variable number of tandem repeats marker. The alleles at the D1S80 locus
(16) were amplified by using the reagents included in the AmpliFLP D1S80 PCR Amplification and Typing Kit (Perkin-Elmer Italia, Monza, Italy). Amplification protocol and interpretation of results were performed according to the manufacturer’s instructions. All 13 pairs of twins with discordant alleles at the D1S80 locus were independently judged to be dizygotic according to the questionnaire administered, thus supporting the validity of the method of determination of the twins’ zygosity applied in the study.
Twin pairs were investigated for the presence of anxiety disorders and family history of panic disorder by two experienced psychiatrists, who were blind to the results of the 35% CO
2 challenge of both twins and the diagnostic assessment of the co-twin. Twins’ diagnoses were made by using the anxiety disorders section of the National Institute of Mental Health Diagnostic Interview Schedule, version III, revised
(17), and data obtained were reevaluated by the same psychiatrists to generate DSM-IV diagnoses. In addition, the presence of sporadic unexpected panic attacks was assessed according to the DSM-IV definition of a panic attack. Information on history of panic disorder in first-degree relatives of twins was obtained by using a modified version of the Family History Research Diagnostic Criteria
(18,
19), adapted to generate psychiatric diagnoses according to DSM-IV criteria. Consensus diagnoses for first-degree relatives of each pair of twins were obtained after a discussion of the information obtained from each twin of a pair.
Data were analyzed by Student’s t tests and chi-square analyses. To analyze concordance rates between monozygotic and dizygotic pairs of twins, the “probandwise” method was applied and rates compared calculating z scores for differences between concordance rates.
Procedure
Two gas mixtures were used: compressed air (placebo) and a mixture of 35% CO2 and 65% O2. Both gases were inhaled through the same self-administration mask connected to a respirometer used to measure the vital capacity of each subject and the volume of gas delivered in each inhalation.
The same day of the diagnostic evaluation, each twin underwent the 35% CO
2 challenge in a double-blind, random, crossover design, according to the method developed by Griez et al.
(1) and described in detail elsewhere
(20). Twins were informed that they would be inhaling two harmless gas mixtures containing different percentages of CO
2 and O
2 and that they might experience some discomfort, ranging from a few neurovegetative symptoms to a definite sensation of anxiety. They were not told that one was compressed air. The possibility of a panic attack was not mentioned in order to avoid any negative cognitive bias related to expectation. The brief nature (a few seconds to 1 minute) of the panic reaction provoked by the inhalation of the 35% CO
2–65% O
2 gas mixture made this decision ethical. After vital capacity was measured, each subject inhaled one vital capacity of 35% CO
2–65% O
2 or of compressed air, in a randomly assigned order, at an interval of 25–30 minutes. At the end of each inhalation subjects were asked to hold their breaths for 4 seconds. We considered the challenge valid only if subjects had inhaled at least 80% of their previously measured vital capacities. Subjects who failed to inhale 80% or more of their vital capacities were invited to repeat the procedure after a resting interval of at least 1 day.
Immediately before and after each inhalation (air or CO
2), anxiety was evaluated by a visual analogue scale for anxiety that described the degree of global subjective anxiety on a continuum from 0 (no anxiety present) to 100 (the worst anxiety ever imaginable). In addition, subjects were asked to score themselves on the Panic Symptom List III-R, a self-rating questionnaire assessing, on a 5-point scale, the 13 panic symptoms described in DSM-III-R
(21) and DSM-IV. Global anxiety reactivity was evaluated as Δ% visual analogue scale for anxiety score (the percent of maximum increment or decrement possible on the visual analogue scale for anxiety; values ranged from –100 to 100)
(20), calculated as follows: 1) if Δ visual analogue scale for anxiety score (post-CO
2 scale values minus pre-CO
2 scale values) was positive, then Δ% visual analogue scale for anxiety score=Δ scale score multiplied by 100 divided by total of 100 minus scale score before CO
2); and 2) if Δ visual analogue scale for anxiety score was negative, then Δ% scale score=Δ scale score multiplied by 100 divided by scale score before CO
2.
According to the scales described and DSM-IV criteria defining a panic attack, the reaction to the 35% CO
2 challenge was considered to be an induced panic attack if it included a sensation of fear or panic with at least Δ% visual analogue scale for anxiety score of 26 or more, an ideal threshold previously shown by a receiver operating characteristic analysis of the 35% CO
2 challenge to separate panic patients and healthy control subjects
(22), and an increment of at least two points for at least four symptoms on the self-rating questionnaire.
The procedure followed for obtaining informed consent for the 35% CO2 challenge was approved by the Human Ethics Committee of San Raffaele Hospital, and, after the participants were given a complete description of the study, written informed consent was obtained.
RESULTS
There were no statistical differences between the two twin groups in age (monozygotic: mean=28.7 years, SD=5.2; dizygotic: mean=28.4, SD=3.7), educational level (monozygotic: mean=14.0 years, SD=3.2; dizygotic: mean=13.6 years, SD=3.1), or sex distribution (monozygotic: five male pairs and 15 female pairs; dizygotic: eight male pairs and 17 female pairs).
Thirty-four twins (37.7%), 18 monozygotic and 16 dizygotic, reacted with an induced panic attack to the 35% CO
2 challenge. In 13 pairs of monozygotic twins and in 15 pairs of dizygotic twins, at least one of the twins reacted with an induced panic attack to the inhalation of 35% CO
2–65% CO
2.
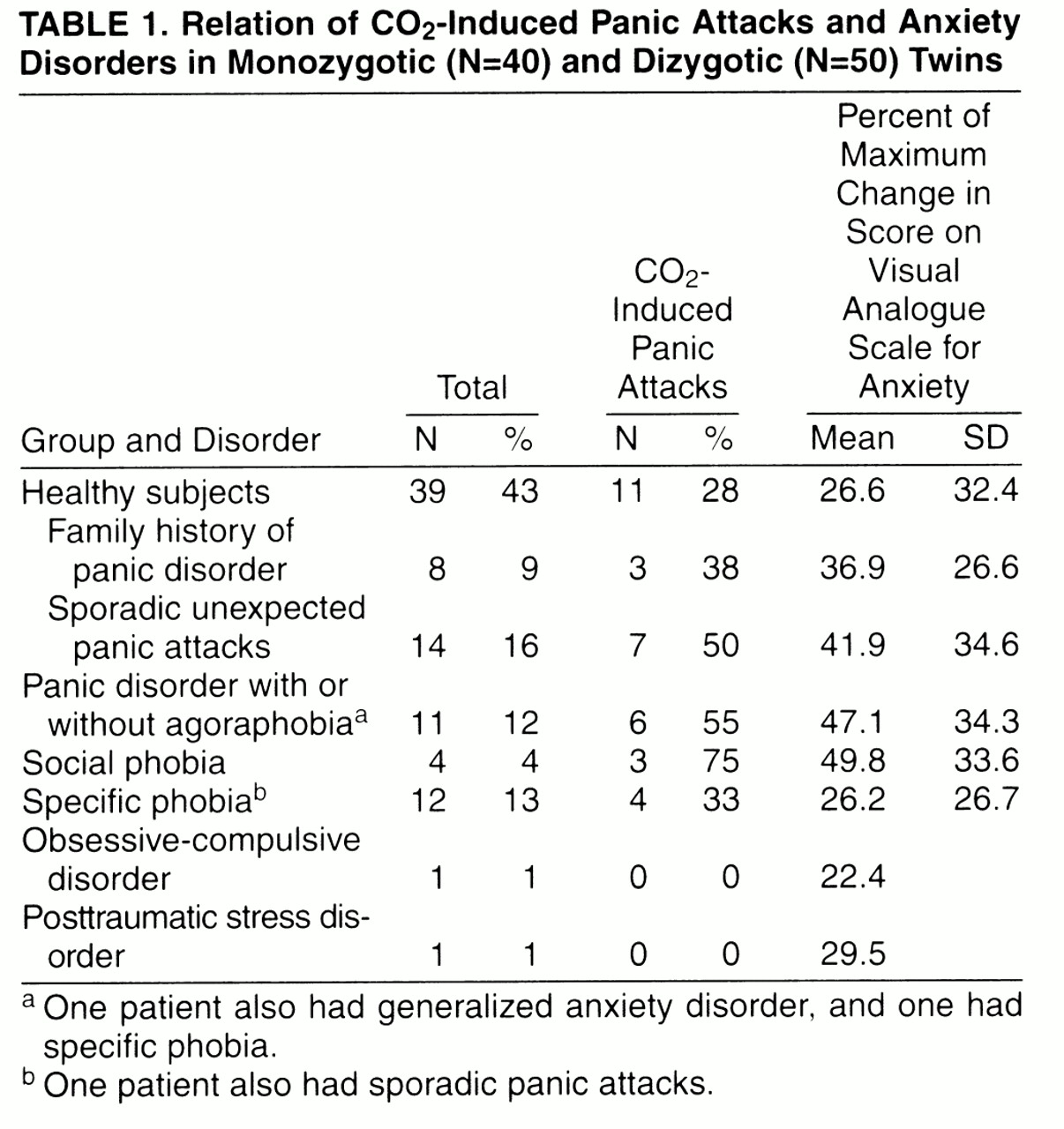
Table 1 reports data on diagnoses of anxiety disorders and sporadic panic attacks in our sample, family history of panic disorder in healthy twins, and the relative rates of induced panic attacks. Of the 11 twins with panic disorder, five (45%) also had agoraphobia. The rate of families with secondary cases of panic disorder in our sample of twins was 17.8% (eight of 45); no significant differences were found between rates in monozygotic (five of 20, 25%) and dizygotic (three of 25, 12%) pairs of twins. Rates of families with secondary cases of panic disorder were 100% (three of three) in pairs of twins concordant for panic disorder, 20% (one of five) in those discordant for panic disorder, and 13.5% (five of 37) in those without panic disorder.
Regarding panic disorder, among monozygotic twins three pairs were concordant and three were discordant, while among dizygotic twins none was concordant and two were discordant. Probandwise concordance rates for panic disorder were higher in monozygotic pairs (six of nine, 67%) than in dizygotic pairs (none, 0%). Regarding spontaneous panic attacks, among monozygotic twins five pairs were concordant and four were discordant, while among dizygotic twins one was concordant and nine were discordant. Probandwise concordance rates for spontaneous panic attacks were higher in monozygotic twins (10 of 14, 71%) than in dizygotic twins (two of 11, 18%).
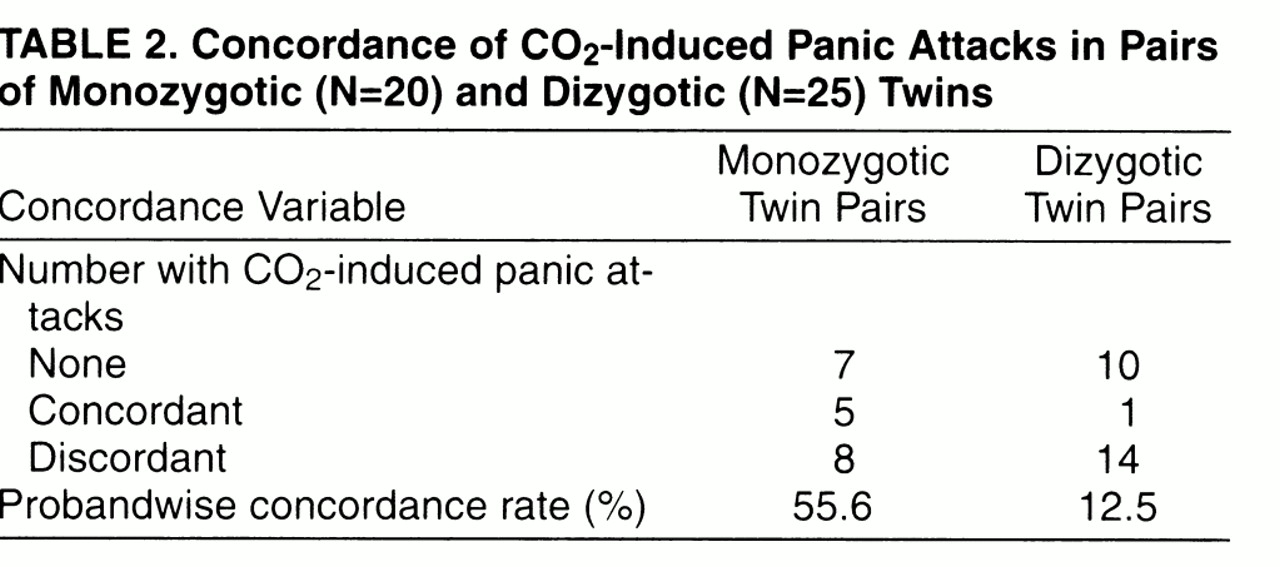
Table 2 reports data on CO
2-induced panic attacks in monozygotic and dizygotic twins. Among monozygotic pairs, five were concordant and eight discordant for CO
2-induced panic attacks, while among dizygotic pairs, one was concordant and 14 were discordant. Probandwise concordance rates for CO
2-induced panic attacks were significantly higher in monozygotic pairs (10 of 18, 55.6%) than in dizygotic pairs (two of 16, 12.5%) (test of the difference between two proportions: z=2.3, p<0.03; 95% confidence interval for the difference=0.109–0.753), with a monozygotic/dizygotic ratio of 4.4.
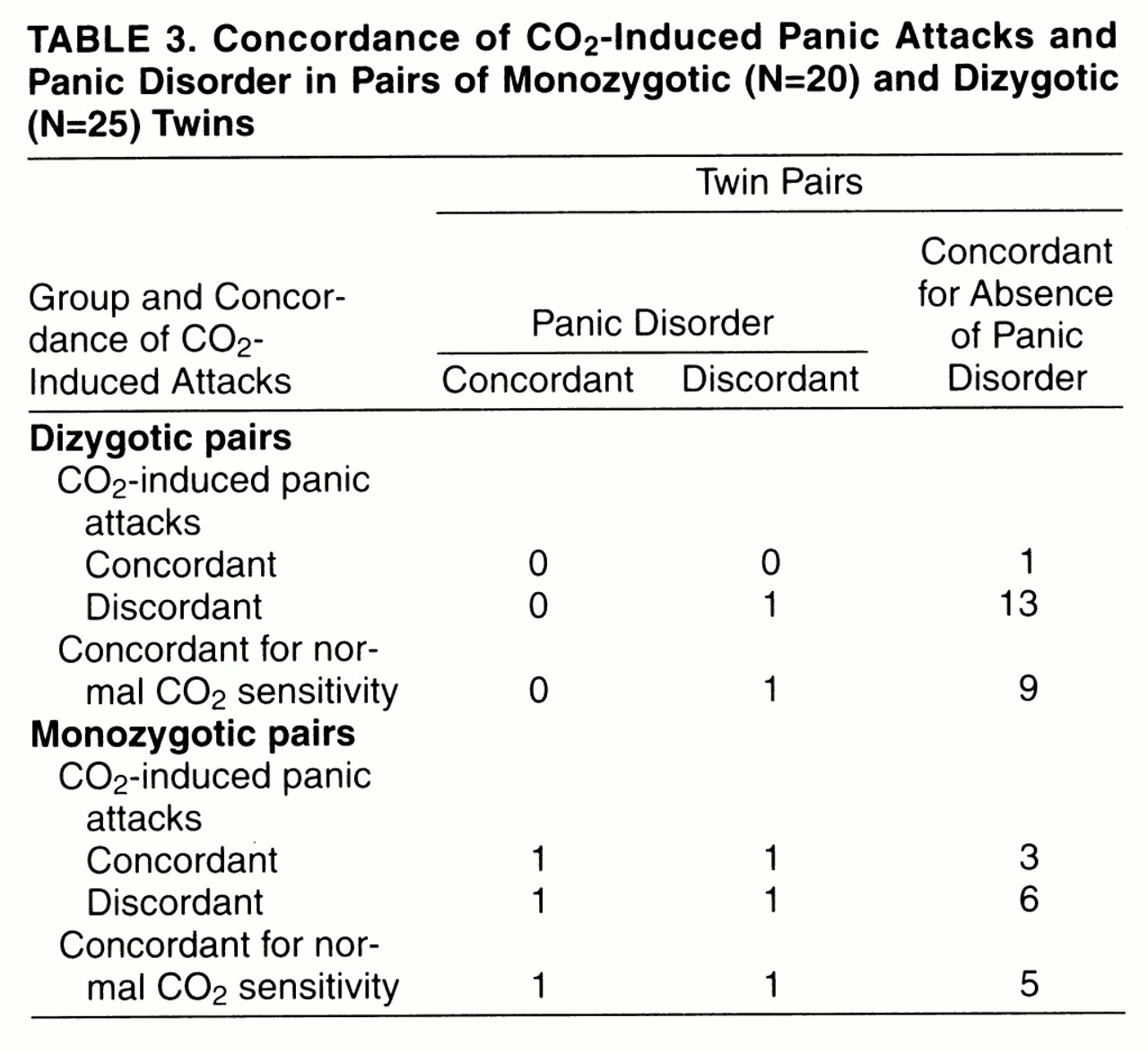
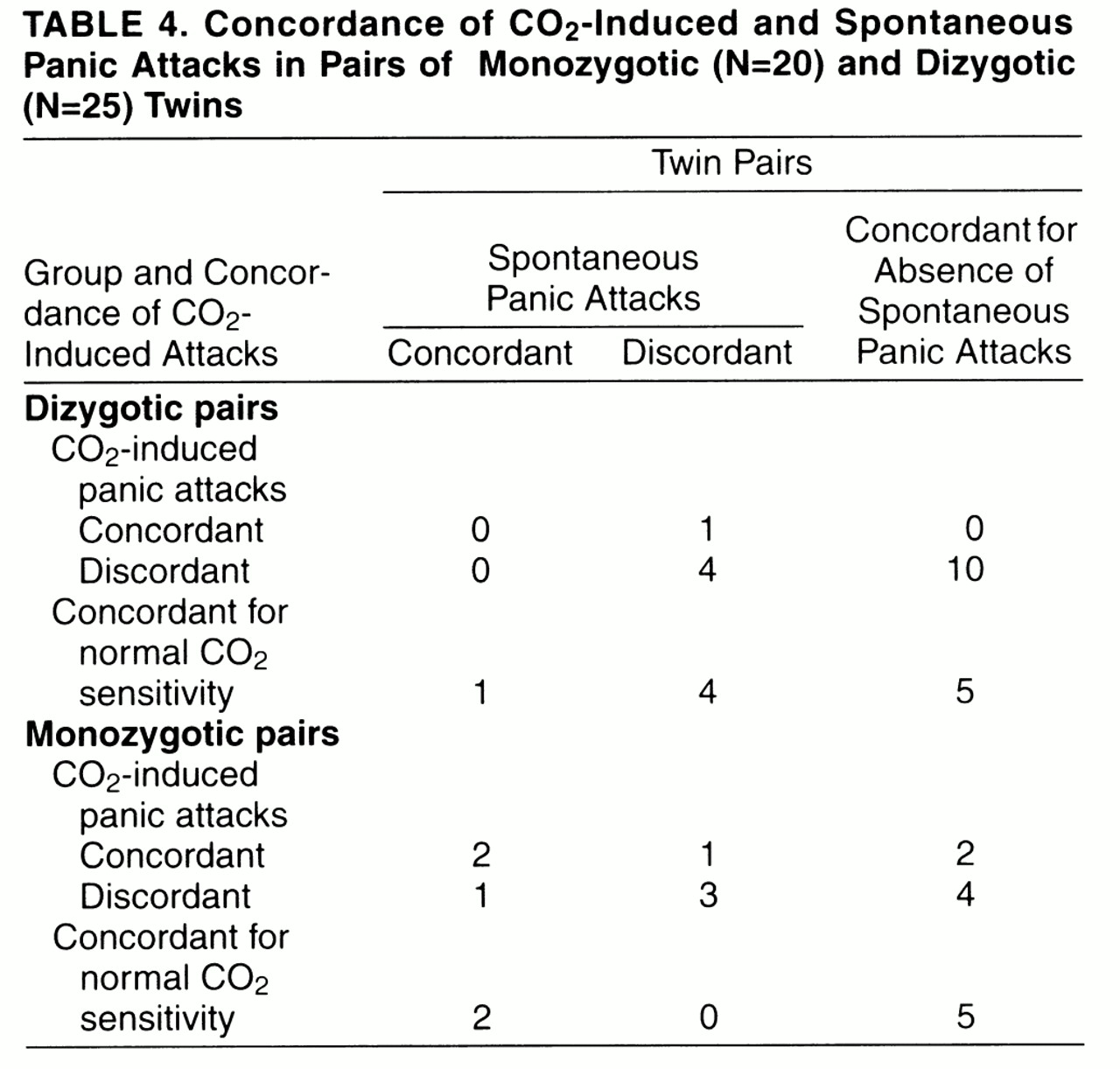
Table 3 and
table 4 report monozygotic and dizygotic concordance rates for CO
2-induced panic attacks and, respectively, personal history of panic disorder and spontaneous panic attacks, either sporadic or recurrent. For the pairs that were concordant for CO
2-induced panic attacks, among dizygotic pairs none was concordant and none discordant for panic disorder, and none was concordant and one discordant for spontaneous panic attacks; among monozygotic pairs one was concordant and one discordant for panic disorder, and two were concordant and one was discordant for spontaneous panic attacks. Twins’ probandwise concordance rates for panic disorder and spontaneous panic attacks among those concordant for CO
2-induced panic attacks were, respectively, two (67%) of three and four (80%) of five for monozygotic pairs and none and none (0%) of one for dizygotic pairs;among pairs with normal sensitivity to CO
2, rates were two (67%) of three and four (100%) of four for monozygotic pairs and none (0%) of one and two (33%) of six for dizygotic pairs.
DISCUSSION
The present study addresses the role of inherited and noninherited components in 35% CO2-induced panic attacks. The results of this study might be considered preliminary, since the sample of twins examined was not very large. Some preliminary observations should be reported.
The prevalence of panic disorder in our sample (12%) is higher than the prevalence reported for general populations
(9), possibly as the result of the way in which subjects were recruited, which probably made subjects who had anxiety symptoms more interested in participation in the study. The prevalence of sporadic panic attacks was similar to the prevalence reported for general populations
(9,
23).
The CO
2-induced panic attack rate of 28% among healthy subjects in this sample seems to be higher than values reported in previous studies
(4,
6,
20) and near that for those with a family history of panic disorder (38%). It should be noted, however, that in the present study the sample examined was composed of twins, who are not independent subjects; in the other studies the groups of healthy subjects were composed of independent subjects. Thus, rates obtained in this study are not comparable to those from our previous studies. In addition, the sample of healthy subjects with a family history of panic disorder was very small (N=8), making a type II error possible.
Probandwise concordance rates for 35% CO
2-induced panic attacks were significantly higher in monozygotic (55.6%) than in dizygotic (12.5%) pairs of twins, demonstrating that heritability contributes substantially to the susceptibility to react to the inhalation of hypercapnic gas mixtures with a panic-like episode. The plausibility of this idea is supported by the evidence of a relevant role of genetic factors both in panic disorder
(11-
14) and in chemosensitivity
(24-
26). It should also be recognized that although monozygotic pairs show a higher concordance rate than dizygotic pairs, half of the monozygotic pairs of twins were discordant. This finding suggests that nongenetic biological/environmental factors may also play an important role in the development of CO
2-induced panic attacks.
To our knowledge, this is the first study to examine the role of genetic factors in the susceptibility to panic-provoking procedures. The observation of a relevant role of genetic factors in CO
2-induced panic attacks might suggest that the recently reported association between CO
2 hyperreactivity and panic disorder
(6-
8) might be genetic in nature.
According to
table 1, in the present sample, CO
2 hypersensitivity seems to be not specifically related to the diagnosis of panic disorder but also a characteristic of other anxiety disorders (social and specific phobia) and of a relevant sample of nonclinical subjects. Thus, hypersensitivity to CO
2 might be considered not a simple marker of panic disorder but the expression of a vulnerability to a spectrum of disorders, whose main clinical expression might be represented by panic disorder.
Similar to CO
2-induced panic, concordance rates for panic disorder were higher in monozygotic than in dizygotic pairs of twins, confirming data previously reported in the literature
(13,
14). It should be observed, however, that there was only a partial overlap between CO
2-induced panic and panic disorder among monozygotic pairs of twins, and thus, at least some of the genetic factors involved in these two phenotypes might be different. In addition, the higher concordance rates for panic disorder and spontaneous panic attacks in monozygotic than in dizygotic twin pairs were present both in pairs concordant for CO
2-induced panic attacks and in those concordant for normal sensitivity to CO
2, confirming that there might not be a complete match between the genetic factors of “clinical panic attacks” and those of “CO
2-induced panic attacks” and that other inherited factors, not related to CO
2 sensitivity, might play a role in the pathogenesis of panic disorder.
CO2-induced panic attacks might be considered a phenotypic expression of a genetic vulnerability to panic disorder even before the clinical onset of panic disorder. Formal and molecular genetic studies of panic disorder, in particular studies of families (i.e., segregation analysis), might consider healthy relatives of panic patients hypersensitive to panic as affected members.
CO
2 hypersensitivity might be the expression of an impairment, under genetic control, at some level of the respiratory control system. This impairment might be anatomically related to specific medullary H+-sensitive neurons
(27,
28), as well as to an imbalance of neurotransmitters regulating respiratory control mechanisms. Recent studies
(29,
30) have reported that treatment with antipanic agents (selective serotonin reuptake inhibitors, tricyclic antidepressants) significantly modulates CO
2 hypersensitivity in panic patients. The main neurotransmitters modulated by these antipanic medications have been reported to influence respiration
(31-
33). Serotonergic system activation and α-adrenoceptor or cholinergic receptor blockade reduce CO
2 sensitivity
(33), and it has been claimed that the modulation of these neurotransmitters plays a role in the pathogenetic mechanisms of panic disorder
(34-
37). Finally, it is noteworthy that Bernard et al.
(38) have found “chemosensitive sites in the raphe, the locus ceruleus, the nucleus tractus solitarius, and near the surface of ventro-lateral medulla,” all CNS areas reported to play a significant role in the pathogenesis of panic disorder
(39). Abnormalities at these levels of the respiratory control mechanisms might explain why analogous stimulations of the respiratory centers trigger abnormal responses in panic patients but not in healthy subjects. These abnormalities might be explained by a specific alarm system developed to control for possible risks of suffocation
(40-
42).
Some limitations of the present study must be recognized. The relatively small size of our sample makes a type I error possible. Since we used a cutoff measure of 80% of vital capacity to consider valid the result of the 35% CO2 challenge, below this threshold the amount of CO2–O2 gas mixtures inhaled by each twin of a pair might differ and data obtained might not be completely comparable. Finally, given the method used to determine zygosity of pairs of twins, the possibility cannot be excluded that some pairs might be misdiagnosed as monozygotic or dizygotic.
In conclusion, our findings suggest a relevant role of genetic factors in CO2-induced panic attacks. If these data are confirmed by other studies, CO2 hypersensitivity might become a valid tool in etiologic and genetic investigations of panic disorder.