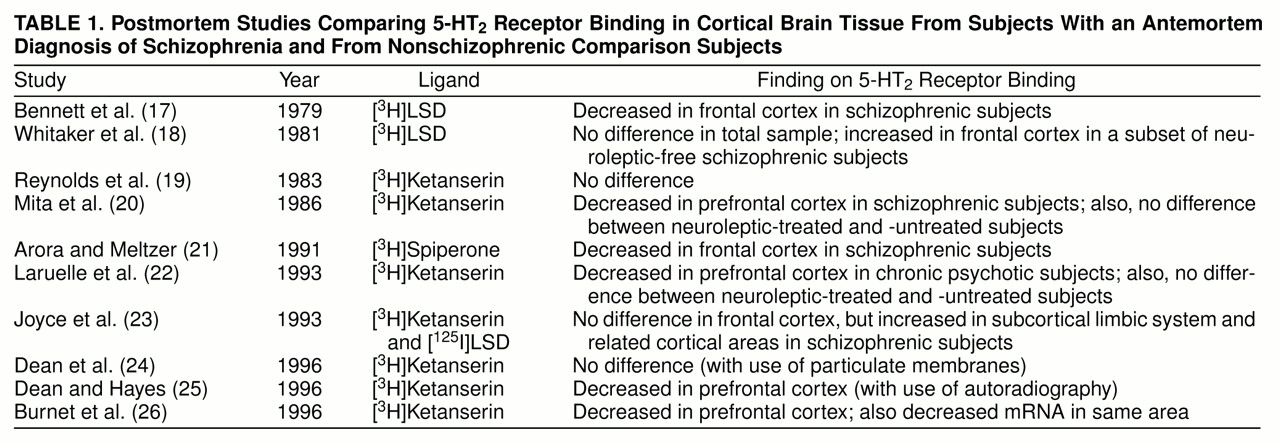
The serotonergic hypothesis of schizophrenia preceded the better-known dopaminergic hypothesis. Following Gaddum’s demonstration in 1953 that LSD, a hallucinogenic drug, had affinity for serotonin receptors, several lines of evidence suggested a role for serotonin in the pathophysiology of schizophrenia
(1–
4). However, the development of effective antipsychotic drugs that were antagonists at dopamine receptors led to a greater focus on and investigation of the dopamine system
(5). With the success of clozapine as an “atypical” antipsychotic
(6), interest in the role of serotonin in schizophrenia has been renewed.
The serotonergic hypothesis is best viewed as complementary to the dopaminergic hypothesis, rather than as an alternative to it, since these systems are anatomically connected and functionally interactive
(7,
8). Several authors have proposed that the atypical antipsychotics derive their unique efficacy at least partly from their dual serotonin-dopamine antagonism, exploiting the neurochemical interaction between these two systems
(2,
4,
7,
9,
10). Despite the recent interest in the role of serotonin 5-HT
2 receptors, the nature of the 5-HT
2 abnormality in schizophrenia, if any, is not known. Until recently, the means to specifically measure 5-HT
2 receptor density in the brains of living patients with schizophrenia were unavailable. Therefore, our current knowledge is derived mainly from studies of 5-HT
2 receptors in postmortem brain tissue and on platelet membranes
(11,
12). The 5-HT system in platelets is developmentally related to the 5-HT system in the brain, but the exact relationship (and, hence, the correlation in disease states) is not completely understood
(4).
Postmortem techniques have demonstrated the presence of 5-HT
2 receptors throughout the cortex and in subcortical structures in normal human brains
(13–
16). Postmortem investigations of cortical 5-HT
2 receptors in schizophrenia are summarized in
table 1.
A review of the literature on postmortem findings suggests a decrease in 5-HT
2 receptors in schizophrenia, but this is not conclusive. While some studies suggest a decrease
(17,
20–
22,
25,
26), others do not confirm this
(18,
19,
23,
24). The reported decrease in B
max (the number of 5-HT
2 receptors) is in the range of 25%–55 %
(17,
20–
22,
25,
26). While there is some inconsistency regarding studies of B
max, there is unanimity regarding the affinity (K
d) of these receptors: no study has reported a significant alteration in the K
d of the receptors in patients with schizophrenia
(17–
26). The previous studies focused mainly on the prefrontal cortex; even so, the different studies focused on different subregions within it. Few studies have investigated other cortical regions (Laruelle et al.
[22] reported on the occipital cortex, while Joyce et al.
[23] reported on the limbic regions), even though 5-HT
2 receptors are uniformly distributed throughout the cortex. Postmortem studies are often confounded by issues of inaccurate diagnosis, different causes of death, different postmortem intervals before tissue processing for analysis, and the effects of concurrent illnesses and medications at the time of death. Some of the studies attempted to address the question of the effect of exposure to neuroleptics, with differing results
(18,
20,
22,
25). To address these shortcomings, we studied 5-HT
2 receptors in neuroleptic-free patients and a group of normal comparison subjects, using [
18F]setoperone and positron emission tomography (PET) imaging
(27–
30).
Setoperone binds to 5-HT
2 receptors with high affinity (K
i∼1 nM). It is relatively selective for the 5-HT
2A subtype, since its affinity for the dopamine D
2 receptor is an order of magnitude lower (K
i∼10–25 nM), and its affinity for the 5-HT
2C receptor is lower still (K
i∼50–80 nM)
(31). It has been shown to bind specifically to 5-HT
2 receptors in both in vitro and ex vivo preparations. When radiolabeled with fluorine-18, setoperone has several features that make it well-suited for PET studies of 5-HT
2 receptors. It rapidly equilibrates across the blood-brain barrier, binds reversibly, and has no metabolites that cross the blood-brain barrier
(32). In PET studies, the cortical signal can be blocked by the competitive antagonist ketanserin and is unaffected by sulpiride, a D
2 blocker, suggesting that [
18F]setoperone binds specifically to 5-HT
2A receptors
(27,
28,
30). Furthermore, the cerebellum is a region almost devoid of 5-HT
2 receptors in postmortem studies
(13,
15), and accordingly, the [
18F]setoperone signal from the cerebellum is not affected by 5-HT
2 antagonists. Since the cerebellum represents free ligand and nonspecific [
18F]setoperone binding
(30), using it as a reference region enables one to obtain semiquantitative estimates of B
max/K
d, indexes of 5-HT
2 binding potential, without arterial puncture or kinetic modeling, making it suitable for routine clinical studies
(33). With the use of these methods, it has been shown that [
18F]setoperone is sensitive to the effects of age on 5-HT
2 receptors, shows robust effects of illnesses such as Alzheimer’s dementia
(34), and is parametrically sensitive to the effects of antipsychotics that bind to 5-HT
2 receptors
(35).
An alternative ligand for the study of 5-HT
2 receptors is [
18F]altanserin. [
18F]Altanserin’s specificity for subcortical 5-HT
2 receptors is slightly higher than that of [
18F]setoperone, since setoperone has moderate affinity for D
2 receptors
(36). However, because D
2 receptors are undetectable in the cortex, the effective specificity for these two ligands is equal for the study of cortical 5-HT
2 receptors
(37). Another radioligand that is used is N1-([
11C]methyl)-2-Br-LSD, which is less selective than [
18F]setoperone or [
18F]altanserin
(37,
38).
At the outset we were interested in two issues. First, on the basis of postmortem data, we hypothesized that the binding potential of [18F]setoperone for 5-HT2 receptors would be decreased in the prefrontal cortex in schizophrenic patients as compared with healthy community volunteers. Second, we wanted to explore any differences in 5-HT2 receptors in nonfrontal regions, since this issue has never been systematically addressed in any previous study.
METHOD
The study was approved by the Human Subjects Review Committee of the University of Toronto. The subjects participated after receiving information regarding the study and providing written consent. Thirteen patients (10 male and three female), whose mean age was 31.1 years (SD=6.9, range=21–43), were included in the study. A 14th patient (male, aged 46 years) participated in the study, but his data were excluded from the analysis because of the poor quality of his PET scan. All patients had a DSM-IV diagnosis of schizophrenia. Patients were assessed by a chart review, discussion with the treating physician, and a clinical interview in which the Structured Clinical Interview for DSM-IV Axis I Disorders
(39) was used. The current symptomatic status of the patients was assessed with the Positive and Negative Syndrome Scale
(40).
The patients were recruited from a university-affiliated psychiatric hospital receiving referrals from a large urban region. The patients’ mean duration of illness, from the first onset of psychotic symptoms, was 4.7 years (SD=3.7 years, range=3 weeks to 10 years) at the time of their scans. Two patients had been ill for less than 6 months at the time of their scans and were therefore provisionally diagnosed as having schizophreniform disorder. In both of these cases, the diagnosis was revised to schizophrenia in subsequent follow-up. Ten patients were neuroleptic-naive, and three were neuroleptic-free. The neuroleptic-free patients had the following neuroleptic-free intervals and prior cumulative exposure to neuroleptics: 7 weeks free with 15 months’ exposure, 4 months free with 4 years’ exposure, and 10 months free with 1 month’s exposure. The previous exposures were accompanied by poor compliance in all cases. Only one patient had received a depot neuroleptic, and that was more than 3 years before the study.
The normal comparison group consisted of 26 subjects (11 male and 15 female) whose mean age was 31.3 years (SD=6.7, range=19–43). These subjects were healthy volunteers recruited from the community by advertisement. They were stratified over the age range of interest (eight to 10 subjects for each decade) to provide a reliable estimate of age effects. They were screened with the Structured Clinical Interview for DSM-III-R—Non-Patient Edition
(41) to ensure that they had no history of psychiatric disorder.
Neither patients nor comparison subjects were currently using any psychotropic medications (with the exception of benzodiazepines for the patients), alcohol, or street drugs. None of the subjects had a history of alcohol or drug dependence, significant head injury, major neurological disorder, or other serious medical illness.
Radiosynthesis and PET Imaging
[
18F]Setoperone was synthesized by a method described previously
(33). PET scans were acquired with the use of a GEMS PC2048-15B head-dedicated PET camera (GE Medical Systems, Milwaukee). Subjects were fitted with a thermoplastic mask to limit head movement. A transmission scan was acquired with a germanium-68 rotating pin source to measure attenuation, prior to a bolus injection of [
18F]setoperone (injected dose range=4.24–5.57 mCi; specific activity range=360–6210 mCi/µmol). PET data were acquired over the next 90 minutes and reconstructed into 22 time frames (five 1-minute frames and then 17 5-minute frames), with 15 6.5-mm-thick axial slices, with the use of a Hanning filter of 5-mm full width at half maximum. Magnetic resonance imaging (MRI) scans were acquired separately with a GE Signa 1.5-T scanner, spin-echo sequence, in an axial orientation, yielding a whole-brain image with 42 3-mm-thick slices. The MRI scans were coregistered with the PET scans by means of the surface-matching algorithm implemented in ANALYZE (CNS Software, Rochester, Minn.).
Data Analysis
Cortical regions of interest were manually traced onto the axial PET images, with the use of Alice 3.0 image analysis software (Hayden Image Processing Group, Denver) implemented on a personal computer-based Windows NT platform, according to a set of conservative anatomical rules based on neuroanatomical landmarks and guided by a standard neuroanatomical atlas
(42). The coregistered MRI slices were used as a visual reference to provide more precise anatomical definition in the drawing of the regions of interest on the PET images. Eight cortical regions of interest were defined: the left and right prefrontal, temporal, parietal, and occipital cortices. A single region of interest was defined that encompassed both hemispheres of the cerebellum. The prefrontal cortical regions of interest were defined on five contiguous PET slices for each hemisphere; the temporal, parietal, and occipital regions of interest were defined on three contiguous slices in each hemisphere; and the cerebellar regions of interest were defined on two contiguous slices. These multiple slices were used in order to reduce partial volume effects. All of the slices within a given region of interest were combined for the analysis. All of the regions of interest were drawn by one of us (R.L.), who was blind to the identity of the subjects.
For each cortical region of interest, a time-activity curve was obtained, reflecting the fluorine-18 activity in the region of interest. The ratio of fluorine-18 activity in the specific cortical region (S) to that in the cerebellum (C) (i.e., S/C–1) at 65–90 minutes was used as an index of 5-HT
2 receptors. The cerebellum was used as a reference region, serving as a measure of free and nonspecific binding of [
18F]setoperone. The time window of 65–90 minutes was chosen because the S/C–1 ratio was stable and showed no statistical change during this window
(33). Theoretically, it can be shown that under such conditions and assumptions, the S/C–1 ratio is linearly proportional to the B
max/K
d of [
18F]setoperone for 5-HT
2 receptors. In addition, Petit-Taboue et al.
(43) have shown empirically that the ratio during this time window is highly correlated (r values=0.91–0.97) with the values of B
max/K
d as obtained from compartmental kinetic modeling. We call this semiquantitative index “5-HT
2R” in further discussions to distinguish it from fully quantitative values of B
max or K
d.
Before this study we had established that this imaging protocol can be repeated in the same individual with high test-retest reliability (intraclass correlation coefficients=0.96–0.98)
(33). Also, we had standardized the technique of data analysis and established high intrarater and interrater reliability (intraclass correlation coefficient, type III: intrarater r>0.98 for author R.L., and interrater r>0.95 for authors R.L., S.K., and C.J.)
(33).
Previous studies
(30,
34,
44–
46) had shown decreases in 5-HT
2 receptors with age, a finding that we confirmed. Therefore, it was planned that age would be a covariate in our analysis. We used two statistical strategies. First, since we had an a priori hypothesis regarding a decrease in these receptors in the prefrontal cortex of patients with schizophrenia, we compared the 5-HT
2R for the two groups by using an analysis of covariance (ANCOVA) with age as a covariate to assess the main effect of diagnosis (i.e., schizophrenia) and to investigate any significant interactions between age and diagnosis. Second, to investigate the 5-HT
2R in other brain regions, we undertook a multivariate analysis of covariance (MANCOVA) with the four major brain regions (frontal, temporal, parietal, and occipital) as dependent variables and age as a covariate. Statistical analyses were done with SPSS version 7.0 for Windows (SPSS Inc., Chicago).
RESULTS
The values of 5-HT2R for the right and for the left regions were not statistically differentiable and were highly correlated for all of the cortical regions (Pearson’s r=0.98–0.99); they were therefore averaged for further analysis. There was no significant effect of sex in the group of normal comparison subjects (ANCOVA: F=1.64, df=1, 22, p=0.21). There were too few women in the patient group (N=3) to permit a valid comparison of the effect of sex on 5-HT2R in the patients. To rule out any bias due to too few female patients, we analyzed the results in the male subgroup only (i.e., the 11 male comparison subjects and the 10 male patients). The results in the male subgroup were no different from those for the complete study group; therefore, we report here a pooled analysis of male and female subjects, comparison subjects as well as patients.
There was no significant difference in activity of [18F]setoperone in the cerebellum (i.e., the denominator “C” in the ratio S/C–1, used to derive the 5-HT2R) between patients and comparison subjects (F=0.60, df=1, 35, p=0.45), indicating that there was no significant difference between the groups in free ligand and nonspecific binding; a difference here could have confounded the interpretation of 5-HT2R and therefore was important to evaluate. Furthermore, there was no significant difference between the groups in the volumes of any of the regions of interest, suggesting that a difference in determination of the regions of interest was not confounding the results.
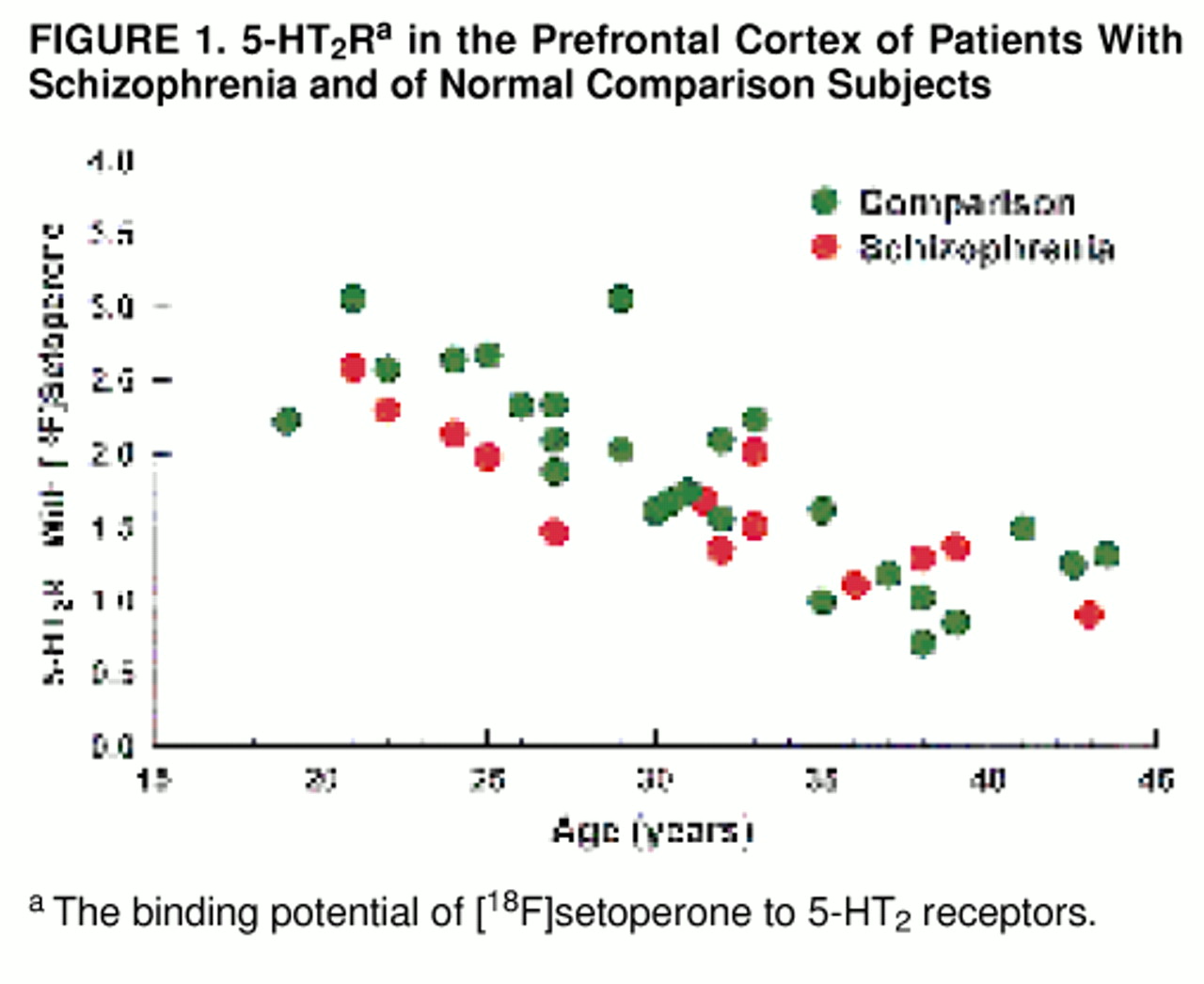
As expected, for the study group as a whole (patients and comparison subjects), there was a substantial decrease in 5-HT
2R with age (Pearson’s r=0.80; F=67.42, df=1, 37, p<0.001) (
figure 1). With age as a covariate, we found no significant effect of diagnosis on 5-HT
2R (i.e., no significant difference in 5-HT
2R between patients and comparison subjects) in the prefrontal region. The mean prefrontal 5-HT
2R was=1.66 (SD=0.50; age-adjusted mean=1.65) for the patient group and 1.85 (SD=0.66; age-adjusted mean=1.85) for the comparison group (ANCOVA: F=1.45, df=1, 35, p=0.24) (
figure 1). While age had a significant effect on 5-HT
2R in both patients and comparison subjects, the age-associated decline was not significantly different between the two groups (ANCOVA: F=0.76, df=1, 35, p=0.39).
The second important question was the effect of schizophrenia on 5-HT2 receptors in all of the brain regions. Since this was an exploratory question, and the regions are highly interrelated, the appropriate method to address this question is a multivariate analysis. A MANCOVA showed an effect of age but no effect of illness. With the four cortical regions as dependent variables and age as a covariate, there was no effect of illness (Pillai’s trace statistic=0.08, and Wilks’s lambda=0.92; F=0.69, df=4, 32, p=0.60). Age itself had a very significant multivariate effect (Pillai’s trace statistic=0.68, and Wilks’s lambda=0.32; F=16.69, df=4, 32, p<0.001), but there was no interaction between the effects of age and illness (Pillai’s trace statistic=0.06, and Wilks’s lambda=0.94; F=0.54, df=4, 32, p=0.71). Quantitatively, the 5-HT2R results were highly correlated across the brain (Pearson’s r values=0.95–0.98), and the values in the different brain regions were as follows: temporal region—patients’ mean=1.79 (SD=0.52; age-adjusted mean=1.78), and comparison subjects’ mean=1.92 (SD=0.68; age-adjusted mean=1.93); parietal region—patients’ mean=1.66 (SD=0.49; age-adjusted mean=1.65), and comparison subjects’ mean=1.80 (SD=0.63; age-adjusted mean=1.80); occipital region—patients’ mean=1.75 (SD=0.41; age-adjusted mean=1.75), and comparison subjects’ mean=1.92 (SD=0.59; age-adjusted mean=1.92).
We found no significant relationship between Positive and Negative Syndrome Scale total score, positive or negative subscale scores, and 5-HT2R for any of the regions of interest, suggesting that differences in 5-HT2R are not associated with profile of symptoms. Finally, three of our patients had received neuroleptics, while the other 10 were neuroleptic-naive. Repeating the MANCOVA in the neuroleptic-naive group, we still found no significant effect of illness (Pillai’s trace statistic=0.06, and Wilks’s lambda=0.94; F=0.45, df=4, 29, p=0.78).
DISCUSSION
This study constitutes the first systematic effort to assess 5-HT2 receptors in schizophrenic patients with the use of neuroreceptor imaging. With [18F]setoperone and PET, the study did not find a significant decrease in 5-HT2 receptors in patients with schizophrenia. These findings are at odds with some of the previous postmortem studies. We now discuss the limitations of postmortem studies, the limitations of our own methods, and the implications of these findings for understanding the role of 5-HT in the pathophysiology and therapeutics of schizophrenia.
Studying neuroreceptors in living patients offers several advantages over postmortem studies. In vitro postmortem studies face confounding variables that may explain some of the inconsistencies in findings between studies
(4,
23,
25,
26). Technical methods vary between laboratories. Postmortem changes in the brain may affect neuroreceptors, depending on the time elapsed between death and preparation of the specimen. Clinical data about the subjects are usually reconstructed retrospectively, and it is difficult to control for clinical variables, especially exposure to neuroleptics before death, which could affect 5-HT
2 receptor binding. None of the postmortem studies included neuroleptic-naive subjects, and the type and degree of cumulative exposure varied greatly. Finally, there are no true “normal” control subjects in postmortem studies, since all subjects have died through some disease process or trauma, factors which could affect 5-HT
2 receptor assessment. The combination of these factors may explain the inconsistency in the literature on postmortem studies and may also explain why our findings are at variance with the postmortem data.
Our study is not without its limitations. We used an index of 5-HT2 receptors that reflects the ratio of receptor density to receptor affinity (i.e., Bmax/Kd) but cannot provide independent measures of either Bmax or Kd. A separate measurement of Bmax would be theoretically desirable. However, it should be pointed out that none of the previous postmortem studies has suggested any change in Kd in schizophrenia. Thus, it is very unlikely that Kd could have obscured a change in Bmax. Furthermore, a change in Kd would hide a change in Bmax only if both Bmax and Kd changed simultaneously, in the same numerical direction, by exactly the same extent—a rather unlikely scenario.
It is important to note that while our data suggest no change in receptor number, they do not address receptor function. The study does not tell us to what extent 5-HT
2 receptors have the same or different functional properties in the two groups. It is of interest that studies using pharmacological challenges which probe the 5-HT system (e.g., the 5-HT releasing agent and reuptake inhibitor fenfluramine or the 5-HT agonist
m-chlorophenylpiperazine) have pointed to abnormal 5-HT function in schizophrenia
(4,
47–
52) or at least in that subgroup of patients who benefit from treatment with 5-HT
2 antagonist neuroleptics such as clozapine
(48,
53,
54). However, the results of these studies are not consistent. Furthermore, an alteration in the neuroendocrine response to a 5-HT
2 challenge does not localize the deficit at the level of the 5-HT
2 receptor, for it can equally plausibly represent postreceptor or primary endocrine system alterations.
Our method of region of interest analysis also has its limitations. While we analyzed more brain regions and more brain volumes than any of the previous postmortem studies, we did not analyze the entire cortex. By choosing five slices for the prefrontal cortex and three for the temporal cortex, and so on, we are reporting on representative regions. Such an analysis lumps together smaller regions within the region of interest, and it is conceivable that if there are very localized changes in schizophrenia (i.e., if the 5-HT2 decreases are limited to a very circumscribed region, for example, just Brodmann’s area 9), we may have missed them. However, there is little uniformity in the postmortem findings that could have focused our analysis further. A voxel-by-voxel analysis of the entire brain space would have been a more sensitive approach for exploring circumscribed changes. While such approaches have been used for comparisons of regional blood flow and are in principle possible for receptor studies, they have not yet been standardized for these purposes. Examining this issue by using a voxel-by-voxel analysis remains a project for the future.
Finally, there is no logical way to “prove” the null hypothesis. The best one can do is to state the certainty with which one can reject a null hypothesis. The postmortem studies showed changes in receptor density of approximately 25%–55%. On the basis of these numbers, we estimated that a study of 12 patients would give us sufficient power to rule out a 25% difference. A post hoc power analysis confirmed our initial assumption. Since there was no difference in the effect of age in the two study groups, and age-corrected variance was similar across the two groups, a post hoc analysis suggests that with 13 patients we had a more than 80% power to detect a 25% decrease at a significance level of p<0.05
(55). Could a decrease smaller than 25% exist? Our data do in fact show a nonsignificant trend in that direction. The patients had a numerical decrease in the range of 10%, but such a change did not reach significance in our study. In fact, if one wanted to design a study to detect a 10% change with reasonable certainty (i.e., greater than 80% power to detect a change at p<0.05), one would need 60–70 patients, a considerable task for any single research site. Thus, while we can rule out a decrease of 25% or greater with reasonable certainty, a smaller change may exist.
The results from this study have significant implications, since it is the first systematic PET study of 5-HT2 receptor density in untreated patients with schizophrenia. The serotonergic hypothesis of schizophrenia holds that 5-HT is involved in the neurochemistry of psychosis. Serotonergic agents such as LSD can induce hallucinations. The literature reviewed in this article, based on postmortem studies, suggests that decreased numbers of 5-HT2 receptors may be the basis of a serotonergic abnormality in schizophrenia. If the negative results from the present study in living patients are correct, then this suggests that a serotonergic abnormality, if it exists, is not at the level of 5-HT2 receptors.
Targeting the 5-HT
2 receptor has been an important factor in the design of the new generation of atypical antipsychotics, all of which possess 5-HT
2 antagonist properties. If there is no abnormality of 5-HT
2 receptor density in schizophrenia and yet 5-HT
2 antagonism is held to confer some unique therapeutic efficacy, the efficacy of 5-HT
2 antagonist drugs must be produced by some indirect mechanism. Such a discrepancy between lack of receptor involvement in disease etiology and a value of that receptor in therapeutics is not new to schizophrenia at all. While it has been difficult to confirm an alteration in dopamine D
2 receptors in schizophrenia, there is no denying a role of these receptors in therapeutics
(56). Such could be the case for 5-HT
2 receptors. In fact, it has been postulated that 5-HT
2 antagonists may exert their effects through a modulation of the dopaminergic system
(7). Thus, while our data argue against a significant decrease in 5-HT
2 receptors in patients with schizophrenia, they do not rule out the role of 5-HT
2 receptors in the therapeutics of schizophrenia.
Our study also shows a dramatic effect of age on 5-HT
2 receptors. In fact, the 5-HT
2 receptor index decreased by about 2%–3% per year in our subjects. Such a decline has been demonstrated by others previously
(30,
34,
44–
46). This decline has potentially important consequences for medication dosage. All current atypical antipsychotics block 5-HT
2 receptors. It is conceivable that as one ages, the receptor reserve declines, and older patients may be more sensitive to the effects of 5-HT
2 receptor blockers. Most clinical trials are focused on adult populations in their 30s or so; thus, there are few systematic data in older age groups. Our finding should alert clinicians to the possibility that the elderly may be particularly sensitive to these medications.
In summary, we failed to find a decrease in 5-HT2 receptors in the prefrontal cortex or other brain regions in schizophrenia. Our results are in contrast to those of the majority of postmortem studies, which show a decrease of 25%–55%. It is not logically possible to “prove” that there is no difference, but our data suggest that if there is any decrease in 5-HT2 receptors at all, the magnitude of decrease is small and may be localized to a small brain region. The results do not rule out the involvement of 5-HT2 receptors in the therapeutics of schizophrenia but suggest that any such benefits are obtained through indirect mechanisms. It will be important to replicate these results with other ligands and with greater statistical power. Future research will also need to be directed at other 5-HT receptor subtypes (in particular the 5-HT1A subtype) and at other levels (e.g., intracellular second messengers) within the serotonergic pathways.