An important physiological role of the neurotransmitter serotonin (5-HT) is behavioral inhibition. Animal and human studies demonstrate that increasing central 5-HT function inhibits aggression
(1,
2); conversely, low 5-HT neurotransmission is associated with impulsive aggressive behavior in rodents, primates, and humans
(1,
3–
5). Indeed, evidence of low 5-HT neurotransmission has been reported to be involved in the etiology of several disorders characterized by behavioral disinhibition, including alcohol abuse or dependence
(6), suicide
(7), bulimia
(8), antisocial personality disorder
(9), conduct disorder
(10), and aggression
(11).
Some alcoholics, particularly those with early-onset alcoholism, have lower levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) in their CSF
(16,
17) and a greater behavioral sensitivity to the 5-HT agonist
m-chlorophenylpiperazine
(18). These individuals may be Cloninger’s type-2 alcoholics, a relatively rare but highly heritable male-limited subtype of alcoholism that is characterized by an early onset of moderate alcohol abuse, regardless of external environment, and frequent criminality
(19,
20). Low CSF 5-HIAA is also associated with a paternal family history of alcoholism in impulsive, violent offenders and fire setters
(21,
22), which suggests the existence of a low 5-HT trait in some of these families.
The present study was designed to test the hypothesis that acute tryptophan depletion would induce behavioral disinhibition and increase aggressive response in men with a multigenerational paternal family history of alcoholism relative to age- and sex-matched comparison subjects. Disinhibition was measured by commission errors in a go/no-go learning task. A modified Taylor aggression task was used to quantify aggression. A heightened sensitivity to behavioral disinhibition in response to a decrease in serotonergic neurotransmission could serve as a vulnerability marker for impulsivity and other disorders of disinhibition (aggression, alcoholism) in this population and be useful for future longitudinal studies investigating serotonergic markers in relation to outcome prediction.
METHOD
Selection of Participants and Baseline Testing
Men, age 18 to 25 years, were recruited through local newspaper advertisements. An initial telephone screen excluded those with significant present or past alcohol abuse or dependence, evidence of a present DSM-III-R axis I diagnosis, significant medical illness, or little knowledge of their biological families. After subjects passed this screen, the study was described, and written informed consent was obtained. These subjects underwent the Structured Clinical Interview for DSM-III-R (SCID)—Non-Patient Version
(25) and the Family History Assessment Module
(26). If necessary, participants were asked to contact family members to obtain further information. The Family History Research Diagnostic Criteria
(27,
28) were used to make retrospective diagnoses of alcoholism in family members up to second-degree relatives. This method has a satisfactory specificity for alcoholism in first-degree relatives but is less sensitive than direct interviews
(29,
30).
The inclusion criterion for individuals at risk for alcoholism was a multigenerational family history of paternal alcoholism, as defined by at least two male alcoholics on the father’s side of the family in two different generations. The inclusion criterion for individuals at low risk for alcoholism was the absence of documented cases of alcoholism in all known first-, second-, and third-degree relatives. Individuals with no family history of alcoholism who had multigenerational family histories of any other axis I disorder were excluded from the study.
Individuals with abnormal ECGs were excluded from the Taylor aggression task. Seven subtests of the WAIS-R
(31) (information, arithmetic, similarities, picture completion, digit span, block design, and digit symbol) were used to estimate IQ
(32). This IQ estimate is highly correlated with WAIS-R full-scale IQs in psychiatric inpatients
(33).
A modified Personal Drinking Habits Questionnaire
(34) (including questions concerning nicotine and drug use) estimated current alcohol and drug use; a caffeine intake survey assessed weekly caffeine intake
(35,
36), and the Michigan Alcoholism Screening Test (MAST)
(37) assessed possible consequences of excessive alcohol intake. The Beck Depression Inventory
(38) provided a baseline measure of depressed mood.
Amino Acid Administration
On the day preceding a test day, participants ate a low-protein diet
(39) and were asked to abstain from alcohol and illegal drug use. On the test day, fasting participants completed the Beck Depression Inventory (38), the Profile of Mood States (POMS)
(40), the Visual Analogue Mood Scale
(41), and the state version of the State-Trait Anxiety Inventory
(42); produced a urine sample to screen for drugs of abuse (by means of the triage panel from Biosite Diagnostics); and provided a blood sample for the measurement of tryptophan levels. The participants received either a tryptophan-free (13 participants with a multigenerational paternal family history of alcoholism and 15 participants with no family history of alcoholism) or a nutritionally balanced amino acid mixture (11 subjects with a multigenerational paternal family history of alcoholism and 18 subjects with no family history of alcoholism) amino acid mixture in a between-subjects, double-blind study. The exact procedure of amino acid administration has been described previously
(39,
43). For both groups with and without a multigenerational paternal family history of alcoholism, the administration of amino acid treatments was randomly assigned in blocks of 10.
For the next 4.25 hours, participants read magazines, watched television, or watched movies (all confined to relatively affectively neutral material). They drank water without restriction and could smoke a limited amount if they desired. No sleeping was allowed.
Modified Taylor Aggression Task
Participants performed the Taylor aggression task
(44,
45) to measure aggressive response in the laboratory. Initially, each participant’s pain threshold was determined by presenting a series of increasing shocks. The task itself was introduced as a competitive reaction-time task. Each participant was instructed to first select a shock level on a panel of eight buttons in front of him to deliver to his opponent should he win the reaction-time trial, each numbered consecutively from one to eight. Following the reaction-time trial, the participant was informed of the opponent’s shock choice by the appearance of one of eight red lights on the panel above each numbered button. The appearance of lights five to eight signified a loss, after which the participant received a shock. Shock levels one to eight increased linearly from 15% to 100% of the person’s given pain threshold. Lights one to four signified a win, and the participant administered the previously chosen shock intensity to his opponent by pressing a button. Participants could monitor shock administrations to their opponents by using a direct-current ammeter to their immediate left. After receiving these instructions, the participants viewed, on a television connected to a videocassette recorder in the adjacent room, a prerecorded videotape of a fictitious opponent receiving the same instructions. This tape served to review the instructions, reinforce the nature of the competition, and present the situation more realistically.
The task consisted of 26 consecutive trials; the first half were under low provocation (shocks administered to the participant ranged in strength from one to four), and the second half were under high provocation (shocks ranged from five to eight). The order of wins and losses and the opponent’s shock choices within the provocation blocks were randomly selected by the computer. All participants received three shocks at each level; they alternately won one trial and lost two trials or won two trials and lost one trial. Participants won and lost half of the trials in both provocation conditions. Dependent variables included the shock intensity chosen before each reaction-time trial, the shock duration and latency to shock delivery on those trials in which the participant administered a shock to his opponent, and the reaction time to the stimulus-by-provocation level (low and high). The first shock intensity, chosen before the first reaction time test, was analyzed separately as a measure of unprovoked aggression.
Go/No-Go Learning Task
After the Taylor task, participants were administered the go/no-go discrimination task
(46,
47). Participants learned by trial and error to press a button for “active” stimuli and not to press for “passive” stimuli. Stimuli consisted of eight two-digit numbers (four active, four passive, which ranged from 03–99) repeated 10 times in different, randomly assigned orders for 80 total trials. Four different sets of eight numbers were employed (one per condition). Correct responses were rewarded with a high-pitched tone, presentation of the word “Correct” on the computer screen, and the addition of 10 cents to an on-screen running tally of the participant’s earnings. Incorrect responses were punished by a low-pitched tone, presentation of the word “Wrong,” and subtraction of 10 cents from the participant’s earnings.
All participants completed four conditions. In the reward-punishment condition, participants began with $1.00. Responses to active numbers were reinforced, and responses to passive numbers were punished. In the punishment-only condition, participants began with $4.00. Responses to passive numbers and nonresponses to active numbers were punished. In the reward-only condition, participants started with no money. Responses to active numbers and nonresponses to passive numbers were rewarded. In the punishment-reward condition
(48), participants began with $1.00; nonresponses to active numbers were punished, and nonresponses to passive numbers were rewarded (see
table 2 in reference
48). Each condition was preceded by a 12-trial reward pretreatment in which the ratio of active to passive numbers was 2:1. This pretreatment hypothetically served to establish a dominant response set for reward
(47,
49).
Participants were given instructions for the go/no-go task, the reinforcement contingencies, and the process of trial-and-error learning. With the experimenter (D.G.L. or a research assistant) present, they completed eight practice trials that involved four presentations of each of two practice stimuli (01 as an active number and 02 as a passive number). The experimenter was not present during the testing. Participants were randomly assigned to one of the 24 possible orders of presentation of the four conditions. The experimenter reentered the room between conditions to explain the demands of the next condition. Dependent measures for this task included commission errors (failures to inhibit responses to passive numbers) and omission errors (failures to respond to active numbers).
Participants completed a short interview to verify the success of the Taylor task deception. The experimenter rated the degree to which each participant was deceived, and he was encouraged to voice his feelings concerning the deception. Next, participants were given a high-protein snack and a 1-g
l-tryptophan tablet to normalize plasma tryptophan levels (if the individual was tryptophan depleted) or to maintain the double-blind status of the study (if the individual received the balanced mixture). The tryptophan preparation that was used is available by prescription in Canada and has not been associated with eosinophilia-myalgia syndrome
(50). Each participant was debriefed regarding the procedure and provided with a detailed information sheet.
Determination of Plasma Tryptophan Concentrations
Plasma-free and total tryptophan levels were measured in blood samples before and 5 hours after acute tryptophan depletion (see reference
39).
Data Analysis
Variables were inspected by group for normality, homogeneity of variance, and outliers. Appropriate transformations were applied to correct for violations of these assumptions
(51) and, where employed, are specified. Demographic characteristics of participants with and without a multigenerational paternal family history of alcoholism were compared by using t tests. Plasma-free tryptophan levels were analyzed by using a two-(risk=having or not having a multigenerational paternal family history of alcoholism) by-two-(treatment=tryptophan-free or balanced mixture) by-two (time=preconsumption or 5 hours post-amino-acid consumption), between-within analysis of variance (ANOVA). For the go/no-go discrimination task, an initial two-(risk) by-two-(treatment) by-four-(condition=reward punishment, punishment only, reward only, or punishment reward) by-two (type of error=omission error or commission error), between-within ANOVA on square root-corrected errors was followed by separate two-(risk) by-two-(treatment) by-four (condition), between-within ANOVAs on square root omission errors and commission errors. Dependent measures on the Taylor aggression task were analyzed by means of separate two-(risk) by-two-(treatment) by-two (provocation level=low or high), between-within ANOVAs. The mood data were analyzed by means of separate two-(risk) by-two-(treatment) by-two (time), between-within ANOVAs. Statistically significant interactions were further analyzed by means of simple interaction effects tests followed by pairwise comparisons by means of the Newman-Keuls procedure. Geisser-Greenhouse corrections were used for all main effects and interactions involving repeated measures. Multiple-regression analyses identified variables that significantly predicted commission errors.
Ethics
All participants provided written informed consent. The study was approved by the Research Ethics Board of the Department of Psychiatry, McGill University. Participants were compensated for lost time.
RESULTS
Demographic Data
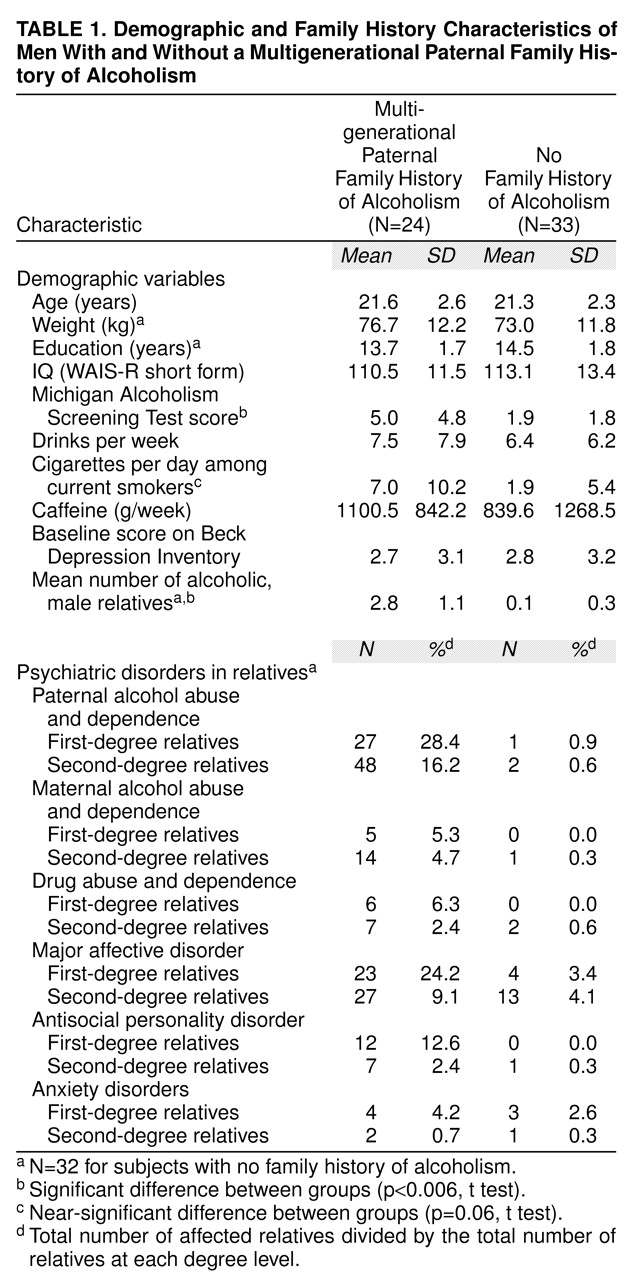
Participants with and without a multigenerational paternal family history of alcoholism did not differ significantly on demographic measures, with the exception of (square root) MAST scores (t=–3.19, df=55, p<0.003) (
table 1). Participants with a multigenerational paternal family history of alcoholism tended toward greater (inverse) smoking frequencies (t=1.95, df=39.76, p=0.06).
Groups differed, by definition, with respect to their family histories of paternal alcoholism but also according to their family histories of maternal alcoholism and major affective disorders. Three men with a multigenerational paternal family history of alcoholism met the criteria for past alcohol abuse, and one met the criteria for dependence for a 6-month period 3 years before testing. Two participants with a multigenerational paternal family history of alcoholism had past histories of major depression, one had a past history of major depression and substance dependence, and one had a past history of cannabis dependence.
Six men with no family history of alcoholism (three given the tryptophan-free and three given the balanced mixtures) and 13 men with a multigenerational paternal family history of alcoholism (eight given the tryptophan-free and five given the balanced mixtures) tested positive for recent use of tetrahydrocannabinol (THC) or amphetamines or both on the triage panel. The frequencies of participants who tested positive were not significantly different between the tryptophan-free and balanced-mixture conditions (χ2=0.88, df=1, p>0.05).
Five participants with a multigenerational paternal family history of alcoholism (four given the tryptophan-free and one given the balanced mixture) (p=0.22, Fisher’s exact test, one-tailed) and one participant with no family history of alcoholism (who was given the tryptophan-free mixture) vomited after amino acid administration, yet they completed the study. Substantial decreases in levels of total plasma and free tryptophan were noted in the participants with a multigenerational paternal family history of alcoholism after acute tryptophan depletion (mean=88.3% depletion of total tryptophan, 85.7% depletion of free tryptophan).
Analysis of Serum-Free and Total Tryptophan Levels
A highly significant treatment-by-time interaction was found for square root, plasma-free tryptophan concentrations (F=449.41, df=1, 53, p<0.0001) (
table 2). The tryptophan-depleted amino acid mixture resulted in a decline in free and total plasma levels of tryptophan of 89% across groups.
Modified Taylor Aggression Task
One individual who was given a balanced mixture who had a multigenerational paternal family history of alcoholism did not participate in the Taylor task because of an abnormally short P-R interval on his ECG. Analysis of log pain threshold levels revealed a significant treatment main effect (F=6.99, df=1, 52, p=0.01). Pain thresholds were lower after ingestion of the tryptophan-free mixture (i.e., greater pain sensitivity) than after ingestion of the balanced mixture.
For the Taylor task itself, 12 individuals (three with no family history of alcoholism who were given the balanced mixture, one with a multigenerational paternal family history of alcoholism who was given the balanced mixture, four with no family of alcoholism who were given the tryptophan-free mixture, and four with a multigenerational paternal family history of alcoholism who were given the tryptophan-free mixture) were excluded from the following analyses because they were judged to have been either doubtful about the presence of their opponent or claimed not to have been deceived at all. Overall, acute tryptophan depletion did not affect aggressive response (shock intensity, duration, or latency to shock administration) in participants with a multigenerational paternal family history of alcoholism in relation to comparison subjects. There was, however, evidence that participants with a multigenerational paternal family history of alcoholism were more aggressive on the task than participants with no family history of alcoholism, because they chose higher shock levels on the first trial (unprovoked aggression) (F=4.06, df=1, 40, p=0.05) and significantly higher shock intensities than did men with no family history of alcoholism under low provocation (risk-by-provocation interaction, F=10.14, df=1, 40, p=0.003). This was contrasted by a significant treatment-by-provocation interaction on log latency to shock (F=4.37, df=1, 40, p=0.04). Under low provocation, latencies to shock were significantly greater in participants who were given the tryptophan-free mixture. Under high provocation, latencies to shock in both groups were significantly lower and not different in magnitude. The results of all analyses were similar when performed on the full group, which suggested that the degree of belief in the deception (as determined during the postexperimental interview) did not affect the results.
Go/No-Go Task
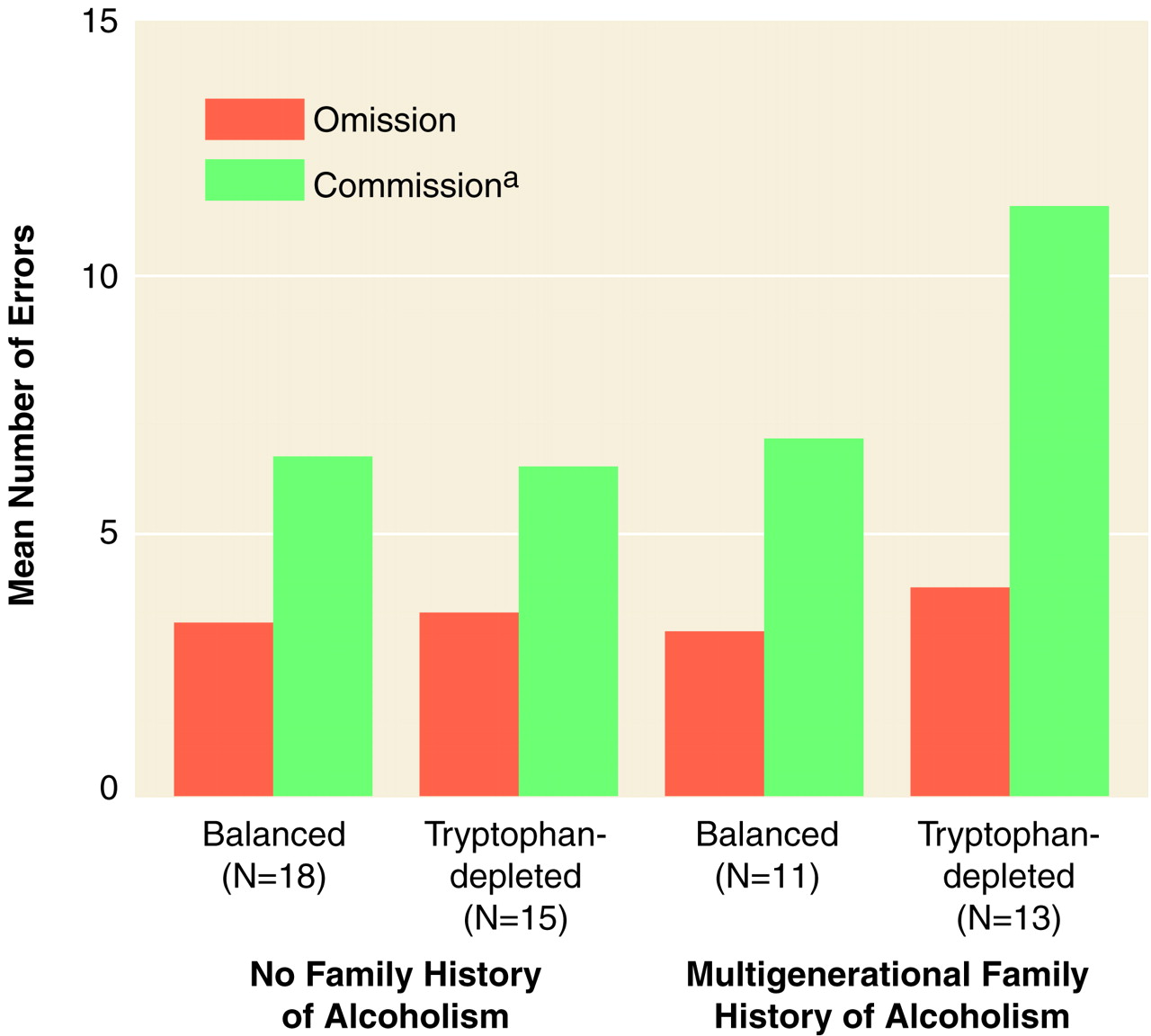
Omission and commission errors were summed across the eight groups of 10 trials within each condition. Errors within the 12-trial, reward pretreatment phase were not included because participants had to be exposed to the stimuli at least once in order to learn which were active and passive. An initial two-(risk) by-two-(treatment) by-four-(condition) by-two (type of error) ANOVA on square root omission errors and commission errors revealed a significant risk-by-treatment-by-error interaction (F=3.95, df=1, 53, p=0.05) (
Figure 1).
Analysis of square root commission errors revealed a significant risk-by-treatment interaction (F=3.94, df=1, 53, p=0.05). Individuals with a multigenerational paternal family history of alcoholism who were given the tryptophan-free mixture made significantly more square root commission errors than did the group with no family history of alcoholism who was given the balanced mixture and the group with a multigenerational paternal family history of alcoholism who was given the balanced mixture and nearly significantly more than the group with no family history of alcoholism who was given the tryptophan-free mixture (group with a multigenerational paternal family history of alcoholism who was given the tryptophan-free mixture, mean=3.03, 95% confidence interval [CI]=2.34–3.72; group with a multigenerational paternal family history of alcoholism who was given the balanced mixture, mean=2.09, 95% CI=1.29–2.90; group with no family history of alcoholism who was given the tryptophan-free mixture, mean=2.06, 95% CI=1.49–2.62; group with no family history of alcoholism who was given the balanced mixture, mean=2.19, 95% CI=1.83–2.56). Effect sizes (differences between the means of the group with a multigenerational paternal family history of alcoholism who was given the tryptophan-free mixture and each of the other groups divided by respective pooled estimates of the population standard deviation) ranged from 0.81–0.91—large effect sizes according to Cohen
(52). There was also a significant condition main effect (Geisser-Greenhouse F=4.15, df=2.94, 155.57, p=0.008). All participants made significantly more square root commission errors in the punishment-reward condition than in the punishment-only and reward-only conditions. There were no interactions involving the condition factor, which indicated that tryptophan-depleted participants with multigenerational paternal family histories of alcoholism made more square root commission errors than the other three groups across conditions. An analysis of square root omission errors revealed a significant condition main effect (Geisser-Greenhouse F=16.02, df=2.7, 143.13, p<0.0001). Participants made significantly more omission errors in the punishment-reward condition than in the other three conditions.
Changes in Mood
In general, significant treatment-by-time interactions were found on many of the mood variables (POMS scores, subscale scores on the Visual Analogue Mood Scale; state scores on the State-Trait Anxiety Inventory), with post hoc tests demonstrating significant increases in the negative mood state in the group who was given the tryptophan-free mixture from the pretreatment phase to 5 hours after consumption of amino acid, whereas the group who was given the balanced mixture showed either no change or a slight improvement in mood.
Variables Predicting Commission Errors
The presence of nausea or vomiting or both after amino acid consumption, a positive urine test for recent drug use, and amount of sleep during the 5-hour wait time were entered into an equation to predict average square root commission errors (i.e., square root commission errors averaged across the four go/no-go conditions). The regression equation was nonsignificant, with simple r2 values for the variables ranging from 0.005 to 0.02. Square root MAST scores and inverse current smoking frequencies failed to predict average square root commission errors. The presence of symptoms or diagnoses of alcohol or drug abuse or dependence, major depression, or anxiety disorders in the participants, coded from the SCID Non-Patient Version, failed to predict average square root commission errors, with simple r2 values again very low. Familial psychopathology, coded from the family history interviews (including a family history of paternal alcoholism, a family history of maternal alcoholism, familial drug abuse or dependence, familial depression, familial anxiety, and familial antisocial personality disorder) did not predict average square root commission errors. Separate regression analyses predicting average square root commission errors by using change (posttreatment minus pretreatment) scores for those POMS and Visual Analogue Mood Scale subscales in which acute tryptophan depletion effects were found did not reach significance.
DISCUSSION
The primary finding of this study, that acute tryptophan depletion increased commission errors on a passive-avoidance learning task in young men at risk for alcoholism, reflects increased behavioral disinhibition in response to an experimental lowering of serotonergic neurotransmission. This result supports the hypothesis that lowered serotonergic functioning may lead to increased impulsivity. This direct experimental evidence complements previous research demonstrating a negative association between impulsivity and 5-HT-related measures
(3–
5,
53). This preliminary finding is in need of replication.
Whereas acute tryptophan depletion significantly increased commission errors on a go/no-go task, it did not affect measures of aggressive response. This may have been due to low statistical power. There was no suggestion of an acute tryptophan depletion effect on any of the aggression measures, however. Alternatively, the absence of an effect may have been due to an insufficient differential between the tryptophan-free and balanced-mixture conditions in tryptophan levels and subsequent 5-HT function. In previous studies, differences in aggressive response were only reported when the tryptophan-free mixture was contrasted with a mixture containing excess tryptophan
(54,
55). That positive results were obtained for impulsivity but not aggression suggests that the laboratory test of impulsivity is more sensitive or that impulsive response is more sensitive to serotonergic modulation. Although acute tryptophan depletion had no effect on aggression, evidence indicates that men with a multigenerational paternal family history of alcoholism were more aggressive than men with no family history of alcoholism, particularly early in the task.
Acute tryptophan depletion caused an increase in negative mood. This change in mood is unlikely to be responsible for the alteration in passive-avoidance response because 1) it was seen equally in both individuals with and without a multigenerational paternal family history of alcoholism, 2) mood did not predict commission errors, and 3) response in the go/no-go task was not influenced by modest changes in mood
(56). It is noteworthy that in this study, the ability of acute tryptophan depletion to elicit a depressive effect was less prominent than in unaffected men at high risk for the development of mood disorders, perhaps because of the higher percentage of first- and second-degree relatives with mood disorders in that study
(39).
Some methodological limitations deserve attention. First, a number of participants with a multigenerational paternal family history of alcoholism tested positive for recent use of THC or amphetamines or both. Recent drug intoxication or withdrawal may have influenced performance on the go/no-go task. However, the proportions of these drugs used by participants in the tryptophan-free and balanced-mixture conditions did not differ. Furthermore, testing positive for recent drug use was not associated with increased commission errors. Additionally, higher frequencies of drug use are characteristic of individuals with a multigenerational paternal family history of alcoholism
(57,
58). A systematic exclusion of these individuals could have resulted in an unrepresentative study group.
Second, participants with a multigenerational paternal family history of alcoholism had higher MAST scores than did participants with no family history of alcoholism. Increased alcohol-related problems in individuals with a multigenerational paternal family history of alcoholism may have affected serotonergic functioning and the behavioral response to acute tryptophan depletion. These variables were not correlated with commission errors, however. As well, individuals with a multigenerational paternal family history of alcoholism tend to show more alcohol-related problems than do individuals with no family history of alcoholism
(57,
58).
Third, a small number of individuals with a multigenerational paternal family history of alcoholism vomited after acute tryptophan depletion. Vomiting may have affected absorption of the amino acid mixture, which reduced the effect of acute tryptophan depletion. These individuals demonstrated substantial reductions in plasma-free tryptophan, however. Emesis may have affected go/no-go performance by lowering mood, yet regression analyses indicated that emesis was not correlated with commission errors.
Fourth, a number of participants were not deceived in the Taylor aggression task. Similar results were obtained when undeceived participants were included in the analyses. This suggests that deception may not be essential in the Taylor test and increases the credibility of the data.
Finally, family histories were collected from the participants rather than directly from their relatives. This method has a high specificity but a low sensitivity (correctly identifying only some of the alcoholic relatives)
(29,
30). Thus, there were probably additional unidentified cases of alcoholism in the relatives of both individuals with and without a multigenerational paternal family history of alcoholism. This argument should work against our hypothesis and, therefore, should not pose a major problem for interpreting the results.
Another factor to consider is the nature of the physiological changes induced by acute tryptophan depletion. Recent results suggest that acute tryptophan depletion substantially decreases human brain 5-HT synthesis
(24) and CSF 5-HIAA
(23). Whether a decline in 5-HT synthesis produces a decline in 5-HT release and neurotransmission remains a working hypothesis. It is also possible that lowering tryptophan levels may lower the levels of other potentially psychoactive tryptophan metabolites, such as tryptamine
(59), melatonin
(60), and quinolinic and kynurenic acids, as well as brain protein synthesis.
Acute tryptophan depletion selectively enhanced commission errors in participants with a multigenerational paternal family history of alcoholism, which suggested that these men are more susceptible than individuals with no family history of alcoholism to an acute lowering of 5-HT neurotransmission. Whether this is due to a preexisting abnormality in serotonergic neurotransmission or to differences in other neurotransmitter systems modulating impulsivity is not known. Preliminary evidence suggests altered serotonergic functioning in sons of alcoholics
(61,
62).
A substantial proportion of the vulnerability to alcoholism is believed to be genetically mediated
(63), particularly in early-onset, male-limited, type-2 alcoholism
(20). Human
(64,
65) and primate
(66) studies suggest a significant genetic component in 5-HT-related measures. Additionally, mice with genetic alterations affecting serotonergic neurotransmission show increased aggression
(67,
68) and alcohol intake
(69). Environmental factors may also be important. Early stressors lead to greater developmental declines in CSF 5-HIAA in monkeys than in unstressed animals
(70). The extent to which low 5-HT function contributes to the genetic risk for alcoholism remains to be determined. The present results suggest that reduced central nervous system 5-HT function may account for some of the behavioral problems of impulse control that characterize individuals with a multigenerational paternal family history of alcoholism. Whether the propensity for disinhibited behavior after acute tryptophan depletion may help predict future outcome (alcoholism or impulsive behavior or both) remains to be investigated.