The selective serotonin reuptake inhibitors (SSRIs) have been widely used for the treatment of depressive disorders since fluoxetine was approved by the U.S. Food and Drug Administration (FDA) in 1987. Several studies have documented the efficacy and safety of fluoxetine (
1–
6). Nevertheless, an article describing six case reports suggested a link between use of fluoxetine and suicidal behavior (
7).
Most reports on fluoxetine come from randomized, controlled clinical trials. While these studies, which are conducted in controlled settings with carefully selected patients, are well designed for evaluation of efficacy over an 8- to 16-week period, they are limited with regard to generalizability. In contrast, naturalistic follow-up studies can shed light on the long-term effectiveness of a medication in uncontrolled settings with a wide variety of patients. However, naturalistic studies lack the rigor required for evaluation of efficacy. Clinical trials focus on internal validity, while naturalistic studies focus on external validity.
Patients who receive treatment in a randomized clinical trial can differ greatly from those in a naturalistic follow-up study. Randomized clinical trials generally have inclusion criteria requiring minimum levels of psychopathology and extensive exclusion criteria, such as psychiatric or other medical comorbidity or current serious suicidal ideation. In contrast, in a naturalistic design, the patients who receive medication include not only the severely ill, such as the actively suicidal, but also the mildly ill, who may receive prophylactic treatment.
The objective of this report was to examine the association between fluoxetine use and suicidal behavior in subjects who were identified as they presented for treatment of an affective disorder at academic medical centers. The analyses in this naturalistic 15-year follow-up study focus primarily on data from the interval during which fluoxetine was available in the United States, approximately the last 8 years of available follow-up. We hypothesized that there would be no elevation in suicidal behavior among patients treated with fluoxetine.
METHOD
The study group came from the 955 probands in the National Institute of Mental Health (NIMH) Collaborative Program on the Psychobiology of Depression (Collaborative Depression Study), but it was limited to the 643 subjects who were followed up after the FDA approval of fluoxetine. They were recruited for the study when seeking treatment for major depressive disorder, mania, hypomania, schizoaffective disorder, depressed, or schizoaffective disorder, manic, at one of five academic medical centers (New York State Psychiatric Institute, New York; Iowa State Psychiatric Institute, Iowa City; Rush Presbyterian Medical Center, Chicago; Massachusetts General Hospital, Boston; and Washington University School of Medicine, St. Louis) from 1978 through 1981. All subjects were at least 17 years of age, white, and English speaking. The methods and objectives of the study have been reported in detail elsewhere (
8). Each subject provided written informed consent before participating in the study. Institutional review board approval was provided at each site.
The standard battery of NIMH Collaborative Depression Study assessments was used in these analyses. The Schedule for Affective Disorders and Schizophrenia (
9) and clinical records were used to determine past and current diagnoses based on the Research Diagnostic Criteria (RDC) (
10). The Longitudinal Interval Follow-up Evaluation (
11) was administered semiannually for 5 years and annually thereafter for assessment of psychopathology, psychosocial status, and intensity of treatment. The Psychiatric Status Rating, which is one component of the Longitudinal Interval Follow-up Evaluation, was used for weekly ratings of severity of affective psychopathology on a 6-point scale (1=asymptomatic, 2=residual/mild symptoms, 3=meets partial criteria, 4=probable episode—missing one criterion symptom, 5=meets full RDC, and 6=meets full RDC with extreme impairment or psychosis).
Information on circumstances of suicide attempts and deaths was recorded. Each suicidal act was rated by a trained mental health clinician with regard to intent (1=none, 2=minimal, 3=ambiguous, 4=serious, 5=very serious, and 6=extreme/expected to die) and lethality (1=no danger, 2=minimal, 3=mild, 4=moderate, 5=extreme, and 6=death).
Tracking Fluoxetine and Other Somatic Antidepressant Treatment
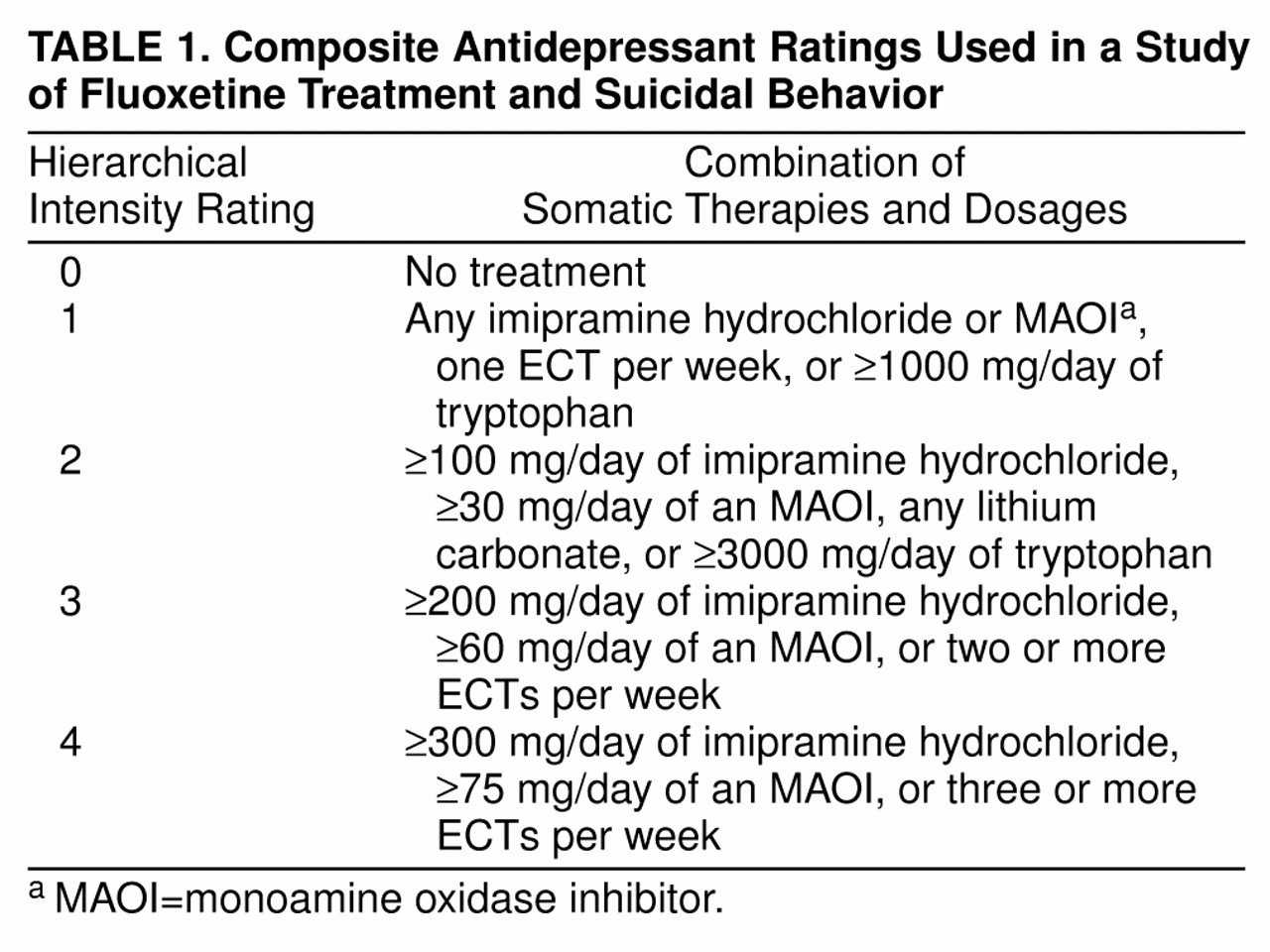
The protocol of the NIMH Collaborative Depression Study did not influence the treatment that the subjects received. Nevertheless, the follow-up interviews recorded the doses and duration of the specific psychotropic medications that each subject received. Wherever possible, subjects’ self-reports were corroborated by office and hospital records. Using these data, we ascertained whether fluoxetine was used during each week of follow-up. In addition, the NIMH Collaborative Depression Study has developed a composite antidepressant rating to serve as a weekly summary measure of the intensity of somatic antidepressant treatment (
12–
14). It rates daily doses of different classes of somatic antidepressant therapies (including lithium carbonate and ECT) on a scale of imipramine equivalence ranging from 0 to 4 (
table 1). Using this information, we classified subjects into one of three mutually exclusive treatment groups: 1) no antidepressant treatment or a nontherapeutic dose (imipramine equivalents of less than 100 mg/day); 2) somatic antidepressant treatments, with imipramine equivalents of at least 100 mg/day (and composite depression ratings of 2 or higher), but not fluoxetine; and 3) fluoxetine.
It might have been interesting to examine risk of suicidal behavior associated with other SSRIs, but they were not commonly prescribed during this follow-up period. Fluoxetine accounted for 95% of SSRI treatment.
Data Analysis
The FDA approved fluoxetine for the treatment of depression on Dec. 29, 1987. For that reason, our analyses primarily focused on the course of somatic antidepressant treatment and suicidal behavior from Jan. 1, 1988, through the end of available 15-year follow-up data. However, initially, the pre-1988 demographic characteristics (age at entry into the NIMH Collaborative Depression Study and gender) and clinical characteristics (age at onset, number of episodes of major depressive disorder, percentage of pre-1988 follow-up time in an episode, and number of suicide attempts during follow-up prior to 1988) of the three treatment groups were compared by means of chi-square tests for nominal variables, Kruskal-Wallis tests for ordered categorical variables, and analyses of variance (ANOVA) for continuous variables. In the analyses described to this point, the group classification was based on treatment received during any time in the post-1987 follow-up. Post hoc tests were performed with the Tukey honestly significant difference test for ANOVAs and the procedure presented by Conover (
15) for Kruskal-Wallis tests.
Subsequent analyses focused on the contemporaneous nature of suicidal behavior and use of fluoxetine or other somatic antidepressant treatments. These analyses incorporated the weekly treatment status, which changed throughout follow-up. The initial analyses were limited to the patients who were treated with fluoxetine. The McNemar test was used for within-group comparisons of the proportion of subjects who exhibited suicidal behavior before the start of fluoxetine treatment with the proportion during fluoxetine treatment.
In this naturalistic design, subjects received varying numbers of trials of antidepressants. Furthermore, the duration of those antidepressant trials varied widely over the course of follow-up. To incorporate this in the analyses, a mixed-effects grouped-time survival model (
16) was examined with the use of MIXGSUR software (
17). In these analyses, treatment was classified into one of the three mutually exclusive categories described above: no antidepressant treatment or a nontherapeutic dose, somatic antidepressant treatment but not fluoxetine, or fluoxetine. Survival time was defined as the number of consecutive weeks during which treatment remained in one of those categories. A survival interval terminated in one of three ways: 1) a suicide attempt or completion, 2) a change in antidepressant classification, or 3) end of follow-up. The last two categories were classified as censored survival intervals. Using this strategy, we were able to examine multiple treatment trials and multiple suicide attempts, in separate weeks, for each subject. The more familiar log rank statistic was not used to compare treatment-specific survival rates over time because the observations were not independent. Instead, the test of the treatment effect in the mixed-effects survival model was used for that purpose. The mixed-effects grouped-time survival model provided a comprehensive analysis of time at risk (i.e., treatment exposure time) for suicidal behavior. Nevertheless, when a subject attempted suicide multiple times in 1 week, only the first of those could be incorporated in the model.
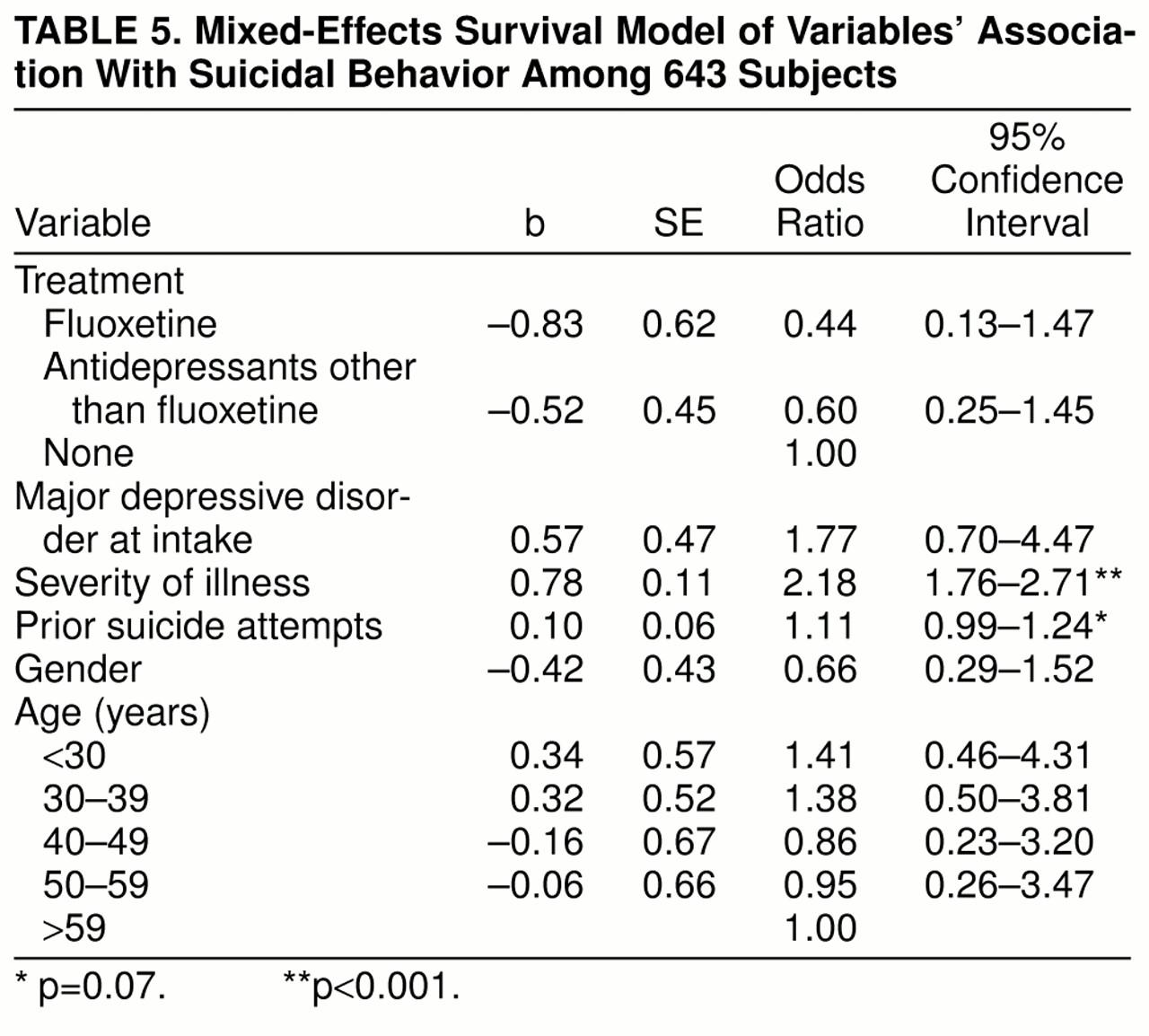
The survival model of suicidal behavior included a random effect to account for correlated observations within subjects, as well as the following fixed effects: weekly status of treatment, number of prior suicide attempts during follow-up, severity of psychopathology, age at intake into the NIMH Collaborative Depression Study, intake diagnosis (major depressive disorder versus other affective disorders), and gender. Severity of psychopathology was defined as the maximum weekly psychopathology rating after 1987 that was recorded prior to the particular survival interval. Age was categorized (<30 years, 30–39, 40–49, 50–59, ≥60) to account for nonlinear risk seen in young adults and the aged. An odds ratio and a 95% confidence interval were calculated for each level of the independent variables; a confidence interval that includes 1.0 indicates that the strength of the association is not greater than would be expected by chance. All statistical analyses were conducted with two-tailed alpha levels of 0.05.
RESULTS
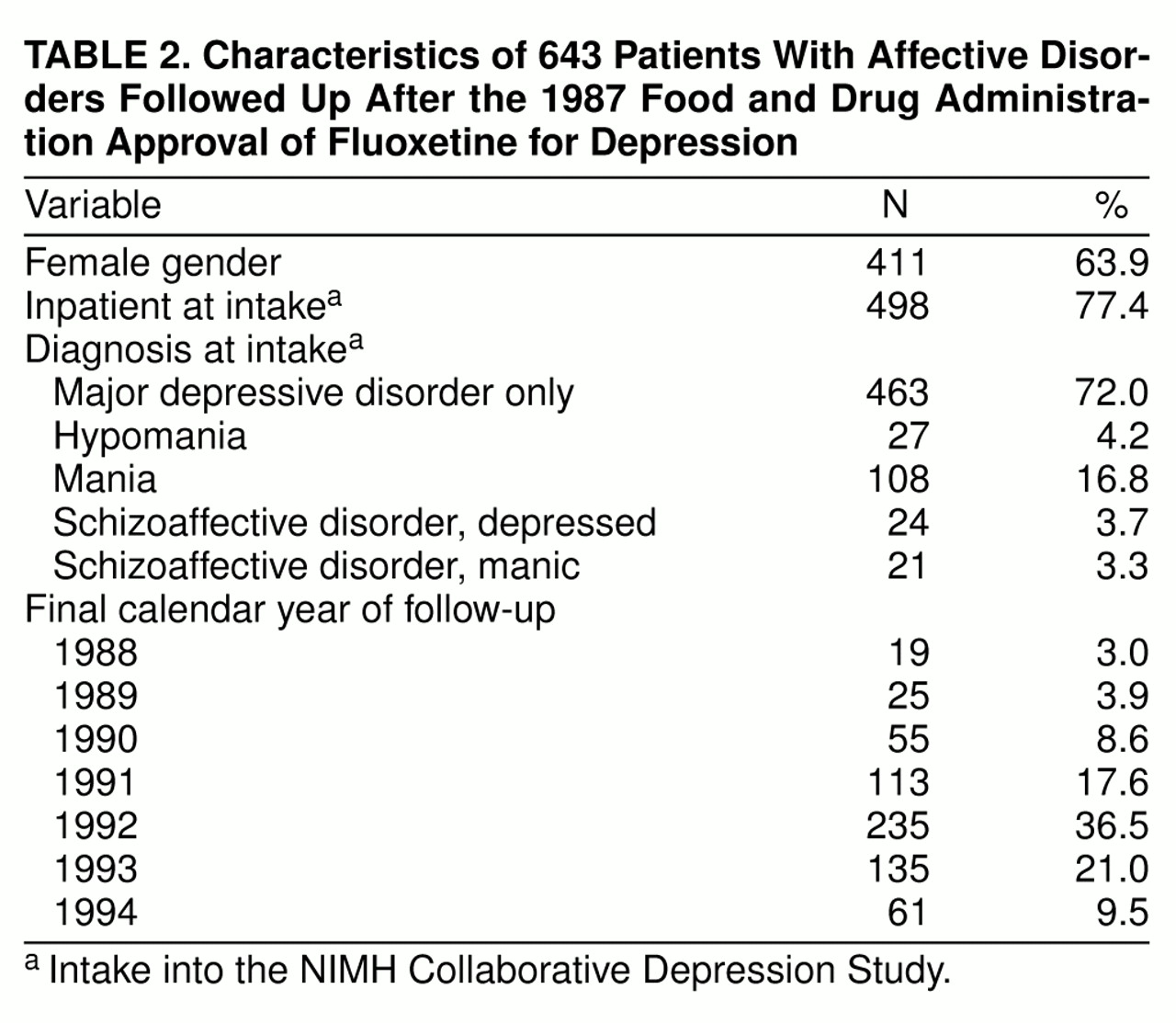
Post-1987 follow-up data were available for 643 (67.3%) of the original 955 probands in the NIMH Collaborative Depression Study cohort. Of the 312 subjects who were not in the post-1987 file, 86 had died, and 28 of these deaths were suicides. An additional 226 probands were not assessed after 1987. The median follow-up time for the 643 probands was 13.0 years from intake into the NIMH Collaborative Depression Study and 4.4 years after 1987. Fifty-one subjects died during the post-1987 follow-up; five of these deaths were suicides. The mean age of the 643 subjects at intake into the study was 38.1 years (SD=14.2). Other characteristics of the study group are shown in
table 2. Seventy-two percent had intake diagnoses of major depressive disorder, and 77% were inpatients at intake into the study. Two-thirds of the study group were last interviewed in 1992 or later.
Somatic Antidepressant Treatments
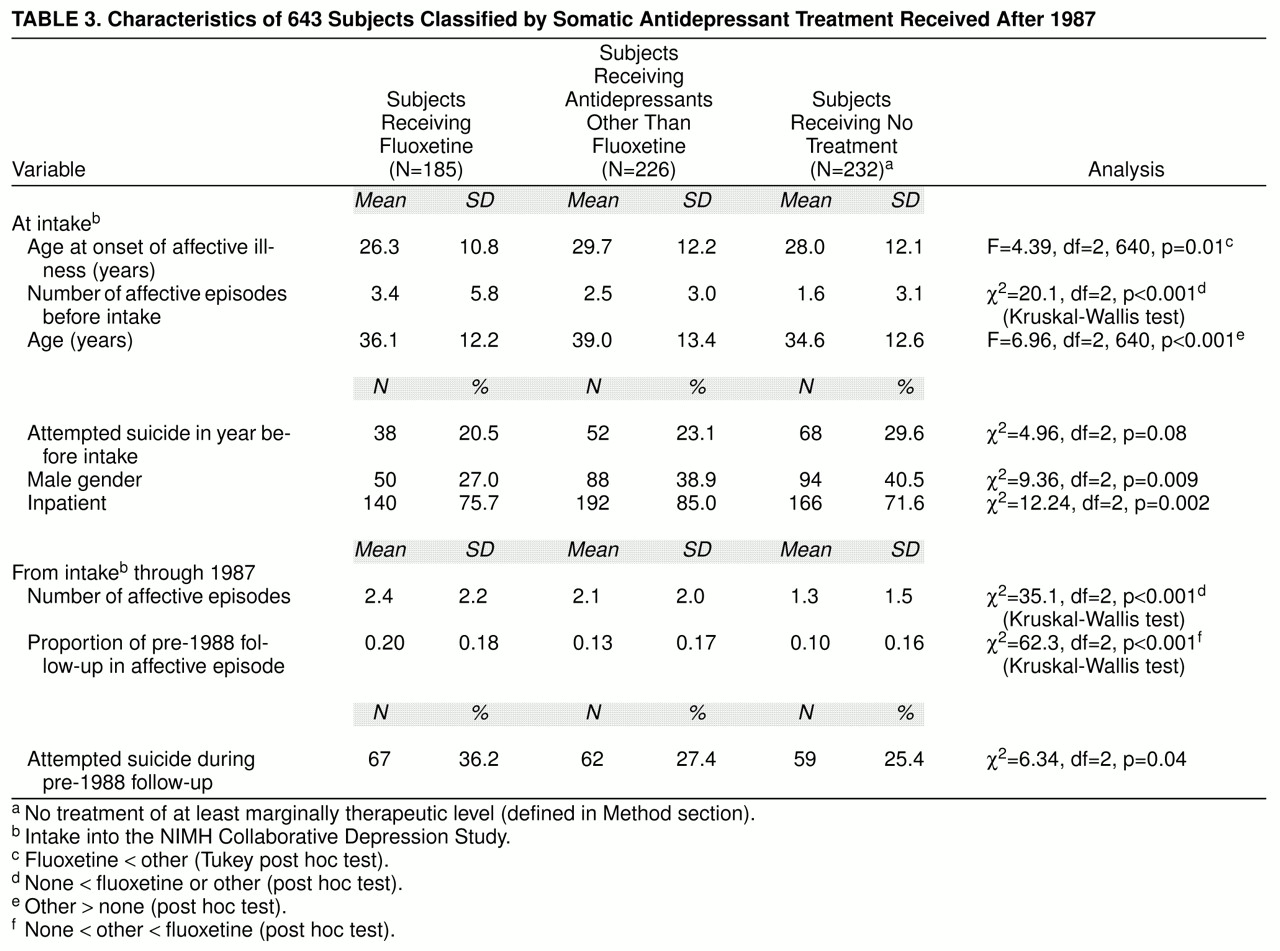
Of the 643 subjects in the study group, 185 (28.8%) were treated with fluoxetine, 226 (35.1%) were not treated with fluoxetine but received other somatic antidepressant treatment (with imipramine equivalence of at least 100 mg/day), and 232 (36.1%) received no such antidepressant treatment during the post-1987 follow-up. Of the 185 subjects treated with fluoxetine, 165 (89.2%) also received at least one other antidepressant at some point during the post-1987 follow-up period.
Fluoxetine treatment
The total number of weeks of fluoxetine treatment ranged from 2 to 270 (mean=55.3 weeks, median=30.0, SD=61.3). Thirty-seven percent of the subjects receiving fluoxetine were treated with the drug for at least 25% of their post-1987 follow-up period.
Other somatic antidepressant treatments
The total number of weeks of treatment with an antidepressant other than fluoxetine during the post-1987 follow-up ranged from 1 to 332 (mean=136.4 weeks, median=140.0, SD=93.6). Seventy-seven percent of the subjects who were treated with another antidepressant received it for at least 25% of this follow-up period.
Characteristics of the three treatment groups
Significantly fewer of the subjects treated with fluoxetine than of those treated with other antidepressants or no antidepressant were men (
table 3). Before fluoxetine treatment, the fluoxetine group had onsets of affective illness at significantly younger ages than those who received other antidepressants, and they reported significantly more prior episodes of major depression at intake into the study than those who received no antidepressants. Nevertheless, similar proportions attempted suicide during the year before study intake (note that these attempts were before fluoxetine treatment.)
In the follow-up years after intake and before 1988, the subjects who later received no antidepressant had significantly fewer affective episodes (
table 3). Those who were treated with fluoxetine spent a significantly higher proportion of time in affective episodes before treatment with fluoxetine. In addition, in comparison with the two groups not treated with fluoxetine, a significantly higher proportion of those who were later treated with fluoxetine attempted suicide after intake into the study and before 1988.
Suicidal Behavior
In this study group the number of suicide attempts that occurred after 1987 ranged from 0 to 15 per subject; 585 (91.0%) had none, 36 (5.6%) had one, 10 (1.6%) had two, and 12 (1.9%) had more than two. In total, 58 subjects accounted for 119 suicide attempts and five completions after 1987. Forty-two (72.4%) of those subjects were known to be in an affective episode at the time of the suicide attempt or completion. The proportions of subjects who exhibited suicidal behavior during post-1987 follow-up, within intake diagnostic groups, were as follows: major depressive disorder only, 9.5% (N=44); hypomania, 7.4% (N=2); mania, 6.5% (N=7); schizoaffective disorder, depressed, 16.7% (N=4); and schizoaffective disorder, manic, 4.8% (N=1).
Suicidal behavior among the subjects treated with fluoxetine
The follow-up interviews of subjects, relatives, and physicians did not include any record of a suicide among the patients treated with fluoxetine. However, the NIMH Collaborative Depression Study routinely examines the National Death Index to identify deaths among subjects. Through this index, one person in the study group who committed suicide was identified whose death certificate indicated that fluoxetine intoxication was the cause of death. It is not clear whether the fluoxetine was prescribed for that subject for treatment of depression or whether it was obtained illicitly for the purpose of suicide. Our own follow-up data indicate that up until 3 weeks before the suicide, that subject had not reported being treated with fluoxetine. However, the NIMH Collaborative Depression Study has no treatment records for the final 3 weeks of her life. It is noteworthy that this subject had made 25 suicide attempts during the course of the 15-year follow-up study; all were before her apparent use of fluoxetine. All of the analyses reported below assume that this subject was being treated with fluoxetine at the time of her death. This approach introduces a bias against our hypothesis that there would be no elevation in suicidal behavior among the subjects treated with fluoxetine.
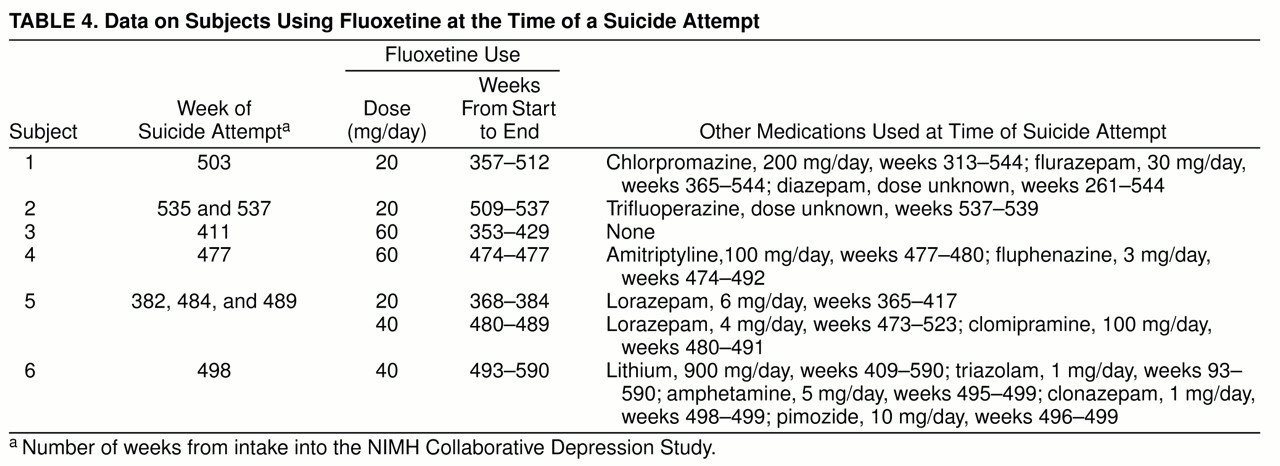
Seven subjects accounted for 11 suicide attempts and the one suicide mentioned above while being treated with fluoxetine. Six of those subjects had intake diagnoses of major depressive disorder, and one had a diagnosis of schizoaffective disorder, manic. Five of the seven were known to be in affective episodes at the time of the suicidal act. As noted above, complete treatment information was not available for the subject who died of fluoxetine intoxication. Of the remaining six subjects who attempted suicide while taking fluoxetine, two were taking 20 mg/day, two were taking 40 mg/day, and two were taking 60 mg/day. Five of them were also being treated with at least one other psychotropic medication at the time of the suicide attempt; three of those five were being treated with another antidepressant. In addition, four of those five were treated with antipsychotics, and three were treated with anxiolytics (
table 4).
Among the subjects treated with fluoxetine, the proportion who exhibited suicidal behavior was significantly reduced from 38.9% (N=72 of 185) during the NIMH Collaborative Depression Study follow-up before fluoxetine treatment to 3.8% (N=7 of 185) during the time of fluoxetine treatment (McNemar’s χ2=56.1, df=1, p<0.001). It should be noted that this analysis ignores the varying durations of treatment. In this group who took fluoxetine (N=185), the mean number of weeks of fluoxetine treatment after 1987 was 55.3 (SD=61.3), whereas, for the same subjects, the mean number of weeks without fluoxetine treatment was 169.5 (SD=75.2). The survival model that is described below accounts for the varying intervals of treatment.
Suicidal behavior among the subjects treated with somatic antidepressants other than fluoxetine
There were 42 suicide attempts and one completion by 23 subjects who were being treated with somatic antidepressants but not fluoxetine during the week of the event.
Suicidal behavior among the subjects not treated with antidepressants
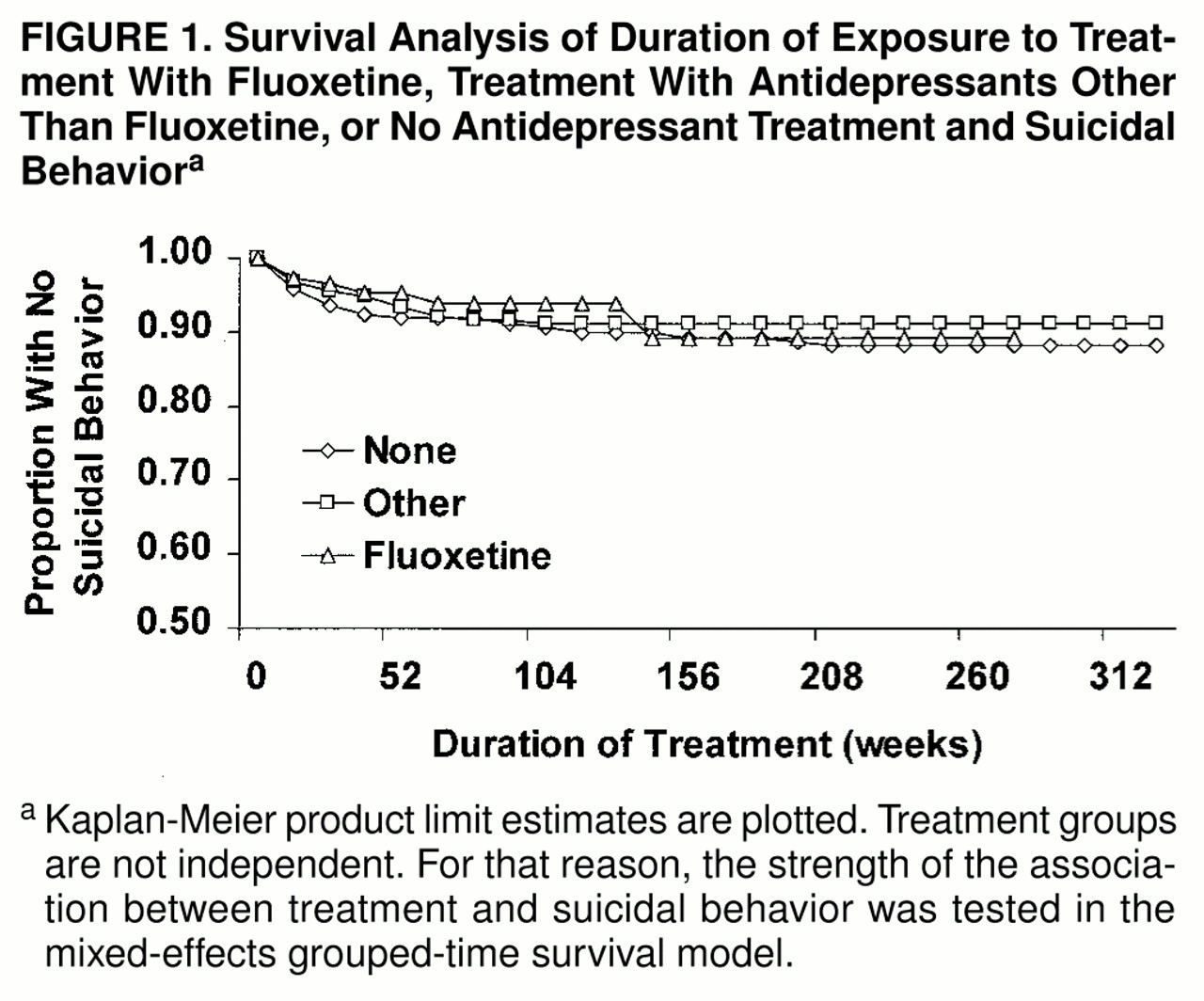
There were 66 suicide attempts and three completions by 39 subjects who were not treated with any somatic antidepressants during the week of the event. Survival curves of treatment exposure time and suicidal behavior are presented in
figure 1, stratified by treatment. Because the treatment groups were not independent, the strength of the association between treatment and suicidal behavior was tested in the mixed-effects grouped-time survival model.
Suicide intent and lethality
Among the subjects who attempted or completed suicide, the ratings of intent and lethality of suicidal acts were compared across the treatments received at the time of the act. These comparisons included only the subjects for whom ratings were available (N=57 of 58, 98.3%). In the case of multiple attempts by the same subject, the maximum intent and lethality ratings for that subject were used. Although the mean level of severity of intent and severity of lethality was highest among subjects who made attempts while not taking any antidepressant and lowest for those who made suicide attempts while taking fluoxetine, these differences were not statistically significant for intent (fluoxetine group: mean rating=2.2, median=1.5, SD=1.9; group taking other antidepressants: mean rating=2.6, median=2.5, SD=1.4; group taking no antidepressant: mean rating=3.5, median=3.0, SD=1.8; Kruskal-Wallis χ2=5.42, df=2, p=0.07) or lethality (fluoxetine: mean rating=3.2, median=2.0, SD=2.2; other antidepressant: mean rating=3.4, median=4.0, SD=1.8; no antidepressant: mean rating=3.7, median=4.0, SD=1.7; Kruskal-Wallis χ2=0.72, df=2, p=0.70).
Mixed-effects survival model
A grouped-time survival model was used to examine the association of post-1987 suicidal acts with fluoxetine treatment, other somatic antidepressant treatments, and other hypothesized risk factors, including gender, intake diagnosis, age at intake, severity of psychopathology, and number of prior suicide attempts. For this survival model, the survival times were grouped into intervals as follows: monthly for the first 3 months, quarterly for the remainder of the first year, semiannually for years 2 through 3, and more than 3 years. These analyses indicated that, relative to no antidepressant treatment, there were nonsignificant reductions in the risk of suicide attempts associated with the use of fluoxetine and with the use of other somatic antidepressant treatments in the absence of fluoxetine. Specifically, fluoxetine was associated with a 56% decrease in risk of suicidal behavior, and other somatic antidepressant treatments in the absence of fluoxetine were associated with a 40% decrease in risk, when gender, age and diagnosis at intake, psychopathology, and number of prior suicide attempts were controlled (
table 5). In addition, severity of psychopathology was strongly associated with an elevated risk of suicidal behavior. Each suicide attempt after intake into the study was marginally associated with an increase in risk of suicidal behavior. Gender, age at intake, and diagnosis at intake were not significantly associated with risk.
DISCUSSION
A naturalistic follow-up study of subjects who were recruited when seeking treatment for affective disorders at academic medical centers was used to examine the association of fluoxetine treatment and suicidal behavior. The results indicate that although fluoxetine was prescribed to more severely ill subjects, their risk of suicidal behavior was not elevated. Instead, there were nonsignificant protective effects of fluoxetine and other somatic antidepressant treatments in the absence of fluoxetine. In addition, the risk of suicidal behavior increased significantly with psychopathology and marginally with number of prior suicide attempts.
These findings are consistent with recent reports that did not show an increase in risk of suicidal behavior among patients using fluoxetine (
18,
19) or paroxetine (
20). We found that the subjects treated with fluoxetine had onsets of affective disorders at younger ages, had had more depressive episodes at intake into the NIMH Collaborative Depression Study, and, before fluoxetine treatment, had more prospectively observed suicide attempts and more severe psychopathology. Moreover, all but one of the subjects who attempted suicide while being treated with fluoxetine were also receiving other antidepressants, antipsychotics, anxiolytics, or a combination of these at the time of the attempt. This utilization of multiple psychopharmacologic agents provides additional evidence that clinicians assessed these patients as being severely ill and difficult to treat. Despite the greater severity and suicidality before the use of fluoxetine, suicidal behavior did not increase with the use of fluoxetine. In further support of our findings, whenever we were faced with a decision about classification of contemporaneous suicidal behavior and fluoxetine treatment, we opted for classification that put our hypothesis at a disadvantage. Classification of one subject’s death as a “suicide while taking fluoxetine” was based entirely on the death certificate; toxicology results were not available. We note that a fatal fluoxetine overdose, in the absence of other substances, is extremely unusual
21).
Our findings are subject to several limitations. First, treatment was not randomly assigned, and the reasons for treatment choice were not clear. Undoubtedly, the causal direction between treatment and psychopathology varied in this study group. Second, a variety of somatic antidepressant treatments other than fluoxetine were classified in aggregate for these analyses. Consequently, efficacy varied within that group of antidepressants. However, they were only included if at least a marginally therapeutic dose was prescribed. Furthermore, although it might be of interest to examine the risk of suicidal behavior that was associated with other SSRIs, such as sertraline or paroxetine, there was an insufficient number (1.1% of the person-weeks of exposure to somatic antidepressant therapy other than fluoxetine) in these NIMH Collaborative Depression Study data for such analyses. Third, follow-up rates are a problem inherent in any long-term naturalistic study. However, two-thirds of the study group was followed for at least 4 years after the FDA approval of fluoxetine. Fourth, although the number of subjects was substantial, it may have been inadequate for the study of a rare event such as suicide. However, the analyses focused on suicide attempts and completions which, in aggregate, were not at all rare. Finally, the data on both treatment and suicidal behavior were based primarily on self-report. Thus, brief intervals of treatment and less serious suicide attempts might have been missed. However, in most cases, supplementary data were obtained from hospital records and physicians and, in the case of death, from the National Death Index.
In summary, in this follow-up study, the association between fluoxetine treatment and suicidal behavior was examined. The study included subjects identified as having a variety of affective disorders who received a variety of pharmacological agents and doses both for acute depressive episodes and for prophylaxis. Our data do not provide empirical support for an increased risk of suicidal behavior among persons treated with fluoxetine.
ACKNOWLEDGMENTS
The NIMH Collaborative Program on the Psychobiology of Depression—Clinical Studies was conducted with the participation of the following investigators: M.B. Keller, M.D. (Chairperson, Providence. R.I.); W. Coryell, M.D. (Co-chairperson, Iowa City); H.S. Akiskal, M.D., J. Maser, Ph.D. (Washington, D.C.); P.W. Lavori, Ph.D., T.I. Mueller, M.D., M.T. Shea, Ph.D., M.G. Warshaw, M.S.S., M.A. (Providence, R.I.); J.A. Fawcett, M.D., W.A. Scheftner, M.D. (Chicago); J. Haley (Iowa City); J. Endicott, Ph.D., A.C. Leon, Ph.D., J. Loth, M.S.W. (New York); J. Rice, Ph.D., T. Reich, M.D. (St. Louis). Other contributors include N.C. Andreasen, M.D., Ph.D.; P.J. Clayton, M.D.; J. Croughan, M.D.; G.L. Klerman, M.D. (deceased); R.M.A. Hirschfeld, M.D.; M.M. Katz, Ph.D.; E. Robins, M.D. (deceased); R.W. Shapiro, M.D. (deceased); G. Winokur, M.D. (deceased); R.L. Spitzer, M.D.; and M.A. Young, Ph.D.