Neuroimaging offers an increasingly powerful method for the development of a scientific psychopathology (
1). Within this overall project, functional brain imaging studies of normal affective processing may contribute to better understanding of pathological mood states. For example, positron emission tomography (PET) studies of normal sadness (
2–
5) have recently been used as tests of Mayberg’s limbic-cortical dysregulation model of clinical depression (
6).
In Mayberg’s own study (
2), induced sadness increased regional cerebral blood flow (CBF) in ventral paralimbic regions (anterior insula, subgenual cingulate) and decreased regional CBF in dorsal neocortical and limbic regions (prefrontal, inferior parietal, dorsal anterior cingulate, posterior cingulate). Similarly, in an early PET study (
3), recall or imagination of personally saddening experiences increased regional CBF in the inferior orbitofrontal cortex. Sadness induced by recalling unhappy memories while viewing unhappy facial expressions has been found to increase regional CBF in the left prefrontal cortex, bilateral anterior cingulate, inferior prefrontal cortex, and hypothalamus (
4). Induction of moods by using photographs of actors posing appropriate facial expressions has been associated with more significant changes in subcortical than in cortical regional CBF: in sad mood, the left amygdala regional CBF increased, while right-sided flow decreased (
5).
To disentangle effects of mood from effects of the procedures used to induce mood, in a recent study (
7) imaging was begun only after mood had been induced. In both elated and depressed moods, regional CBF was increased in the lateral orbitofrontal cortex. Regional CBF was also increased in the midbrain in elated mood. In depressed mood, regional CBF was decreased in the rostral medial prefrontal cortex.
The exact pattern of observed changes in regional CBF has varied across PET studies of induced sadness. Increases in the ventromedial cortex and anterior cingulate have been noted as the most consistent findings (
6,
7). Variations in findings probably reflect the fact that sad mood, like depression, results from coordinated activity of networks with components distributed throughout the brain (
6).
Studies of discrete emotional responses, rather than the diffuse, pervasive affect characteristic of moods, offer one approach to delineating some of those components more precisely. Presentation to elderly subjects of film clips eliciting fear/disgust was found to produce activation within the left orbitofrontal cortex, whereas film clips eliciting happiness produced activation of the entorhinal cortex (
8). A study of video film- and recall-generated emotions of happiness, sadness, and disgust found increased regional CBF in the medial prefrontal cortex and thalamus (
9), with recall-generated sadness being particularly associated with activity in the anterior insular cortex. Pictures eliciting pleasant and unpleasant emotions were found to increase regional CBF in the medial prefrontal cortex, thalamus, hypothalamus, and midbrain (
10), whereas unpleasant emotion was distinguished by increased regional CBF in the occipitotemporal cortex, left parahippocampal gyrus, hypothalamus, and amygdala. A study comparing imagining and planning for an emotional situation (attending one’s mother’s funeral) with planning for a nonemotional situation found emotive processing to be associated with increased regional CBF within the medial frontal gyrus and anterior middle temporal gyrus (
11).
In relation to the production of such specific emotional responses, two distinct routes have been recognized (
1,
12,
13). One route largely involves subcortical structures and generates emotional responses to simple perceptual and associative aspects of stimuli. The second route involves cortical structures and generates emotional responses reflecting the meanings or interpretations given stimuli. Functional imaging studies have often used stimuli likely to activate both of these routes (e.g., pictures of maimed bodies), making it difficult to attribute different components of brain activation to distinct aspects of emotion production. The present study focused specifically on the second, “cognitive” route to emotion. This route is of central importance to cognitive models of emotion underpinning cognitive therapy for emotional disorders (
14) and to related approaches suggesting that emotional response is a function of personal meaning.
The study aimed to identify the neural substrate underlying processing of affect-related meanings in the generation of emotion. A comprehensive information-processing framework, interacting cognitive subsystems (
15,
16), provided the theoretical basis for the subtraction method we used. The interacting cognitive subsystems framework distinguishes between two types of meaning: specific meanings of the kind usually conveyed by single sentences and higher-order schematic meanings derived from patterns of specific meanings and appropriate sensory inputs (as when the “intuitive” meaning of a poem reflects both patterns of specific meanings conveyed by the content of the words and effects of the actual sounds of the words). Interacting cognitive subsystems proposes that higher-order meanings directly mediate generation of emotion by the cognitive route; specific, literal meanings affect emotion only to the extent that they contribute to emotion-related higher-order meanings.
Our research strategy was to present patterns of specific meanings and sensory features that either 1) cohered, so that they accessed emotion-related higher-order meanings generating more emotional feeling than the sum of the component specific meanings and sensory patterns presented separately, or 2) did not cohere, so that any emotional feelings generated would be no more than the sum of the effects of the components presented separately.
Pairs of pictures and verbal captions that either did or did not cohere were presented. By using identical component materials in the experimental and comparison conditions, differences in patterns of brain activity elicited by the two conditions could be attributed to accessing higher-order, affect-related meanings and the generation of emotion, rather than to any differences in sensory, perceptual, lexical, or lower-level semantic processes.
We assumed that accessing higher-order meanings in the generation of both positive and negative emotion involves certain common central processes, whereas the processes involved in the production of actual emotional responses differ between positive and negative emotion. Accordingly, it was assumed that areas of brain activity associated with accessing affect-related meanings could be distinguished from areas associated with actual production of specific forms of emotional response by identifying areas activated in common in the contrasts of both positive and reference caption pairs and negative and reference caption pairs.
In summary, the aim of this investigation was to apply subtraction methodology to identify brain regions involved in the processing of affective meanings in the generation of emotion by the cognitive route. In contrast to earlier studies that used PET, the present study used functional magnetic resonance imaging (MRI) with blood-oxygenation-level-dependent contrast (
17). This offers a noninvasive, repeatable method for imaging brain activity with high temporal and spatial resolution.
METHOD
Six healthy right-handed individuals (three of whom were male), whose mean age was 29.8 years (range=25–36 years), were recruited from the health care staff of the Maudsley Hospital, London. After complete description of the study to the subjects, written informed consent was obtained.
Imaging was carried out in the Department of Neuroimaging at the Maudsley Hospital on a 1.5-T General Electric Signa system retrofitted with an advanced nuclear magnetic resonance operating console. One hundred gradient echo echoplanar images of the whole brain depicting blood-oxygenation-level-dependent contrast (
17), with an in-plane resolution of 3 mm (TR=3 seconds, TE=40 msec) at each of 14 near-axial, noncontiguous 7-mm-thick slices over a period of 5 minutes, were acquired. A high-contrast, high-resolution inversion recovery echoplanar image (TE=74 msec, TI=180 msec, TR=16,000 msec) with in-plane resolution of 1.5 mm and 3-mm slice thickness was also acquired from each subject.
Affect Elicitation
Subjects viewed projected color pictures below each of which was a caption. The pictures themselves were relatively unevocative of affect; their ability to elicit affect was manipulated by using either captions that meshed with the content of the picture to elicit positive or negative feelings (experimental images) or captions irrelevant to the content of the picture (reference images), thereby eliciting little feeling.
Experiments 1 and 2 involved comparison of negative and positive images, respectively, with reference images. Within each experiment, across subjects, the same set of pictures and captions was used in experimental and control conditions. Conditions differed in the relationship between captions and images; thus, for example, one subject viewed a negative experimental image composed of a picture of a staircase and the caption “Where she broke her neck,” whereas another subject viewed a control image made up of the same picture and the caption “He is being exploited.” For the first subject, this latter caption was paired with a picture of a man tilling a field, to create a further negative experimental image. One-half the subjects viewed any given picture or caption as part of an experimental pair; the other half viewed it as part of a control pair. In this way, experimental and control conditions included identical pictures and captions and differed only in the relationship between them.
In experiment 3 only meshing pairs of pictures and captions were used in a comparison of images evoking positive feelings and images evoking negative feelings (e.g., a picture of a concert orchestra standing to receive applause with the caption “A triumphant finale” and a picture of a young girl with the caption “She has leukemia and will die within the year”).
Pictures and captions were chosen after extensive pilot testing. During scanning, each image was presented for 9 seconds, with a 1-second blank interval between images. Three consecutive images of one condition alternated with three consecutive images of the alternate condition in repeating 30-second cycles for 5 minutes. The order of experiments 1, 2, and 3 was counterbalanced across subjects.
Immediately after emerging from the scanner, subjects rated each picture-caption pair on a 5-point scale according to how much positive or negative feeling it elicited (none at all=0, slight=1, moderate=2, very much=3, extreme=4). Eight further subjects also rated feelings elicited by picture-caption pairs on the Affect Grid (
18), on which pleasantness of feelings is rated on a 9-point dimension (1=extremely unpleasant feelings, 5=neutral, 9=extremely pleasant feelings).
Image Analysis
After correction for effects of movement by realignment and regression of each realigned time series on the vector of estimated translations and rotations in three dimensions (
19), the time series data at each voxel were fitted to a sinusoidal regression model consisting of sine and cosine terms at the fundamental frequency (frequency of alternation of the two experimental conditions) and at the first and second harmonics of this frequency (
20). The fitting process consisted of two stages and was designed to ensure that significant autocorrelations were removed from the residuals before estimation of model parameters and their standard errors (
20,
21). Failure to do this would lead to underestimation of standard errors and raise the possibility of an inflated type I error rate. The test statistic computed after this fitting process was the fundamental power quotient, which is the power of the response divided by its standard error. The choice of this statistic is appropriate given the fact that the model fitted is essentially a truncated Fourier series, and the power of the response at a given frequency is the parameter of interest.
Data randomization, if conducted with appropriate care, represents an excellent method of producing a null distribution for statistical testing (
22), and we used it in our statistical analysis. Each observed functional MRI time series was randomly permuted 10 times, and the fundamental power quotient was reestimated after these permutations. This resulted in 10 parametric maps of fundamental power quotient estimated under the null hypothesis that the fundamental power quotient was not determined by the periodic experimental design. The two-stage model estimation process described above and in detail elsewhere (
20,
21) ensures that the potential problem of residual autocorrelations in the observed but not the randomized time series—which would otherwise lead to problems in the application of randomization in this context—is addressed. This has been confirmed by direct experiment in which fundamental power quotients were estimated when no periodic stimulation paradigm was used, producing an experimentally defined null distribution. It has been shown that this distribution is very well approximated by randomization both in individuals (
20) and in groups (
23).
All parametric maps were then registered in the standard space of Talairach and Tournoux (
24), as described previously (
23). After spatial normalization, the observed and randomized fundamental power quotient maps were identically smoothed by using a Gaussian filter of 3-mm standard deviation or a full width at half maximum of 7.2 mm, the latter being the effective image resolution after smoothing. This smoothing process accommodates variations in gyral anatomy between individuals and displacement errors during spatial normalization. Robust generic activation was then decided by comparing the median value of the observed fundamental power quotient at each voxel location with the null distribution of median fundamental power quotients obtained from the randomized fundamental power quotient maps. Estimation of median fundamental power quotient was carried out only at the voxel locations in standard space at which every individual had a nonzero fundamental power quotient value. In the current study, this yielded approximately 22,000 median fundamental power quotient estimates in the observed images in standard space and 10 × 22,000 median fundamental power quotients estimated from the identically transformed randomized fundamental power quotient data, thus giving a null distribution size of 220,000. With the use of this distribution, an observed median fundamental power quotient was considered to represent significant activation, with a probability of false positive activation equaling alpha if the median fundamental power quotient observed at that voxel exceeded the (1–alpha) * 100th percentile value of the null distribution. Thus, a voxel is judged as activated with a p value of <0.0005 if the fundamental power quotient exceeds that of the 99.95th percentile of the null distribution.
RESULTS
Ratings after scanning indicated that picture-caption pairs evoked feelings as intended. In experiment 1, negative pairs elicited a mean negative feeling rating of –1.60 (range=–1.00 to –2.00), and control pairs elicited a mean negative feeling rating of –0.21 (range=0.00 to –0.43) (t=6.50, df=5, p=0.001). In experiment 2, positive pairs elicited a mean positive feeling rating of 2.17 (range=2.00 to 2.60), and control pairs elicited a mean positive feeling rating of 0.27 (range=0.00 to 0.73) (t=7.72, df=5, p<0.001). In experiment 3, the mean positive feeling rating of positive picture-caption pairs was 2.57 (range=1.47 to 3.33), and the mean negative feeling rating of negative pairs was –2.10 (range=–1.43 to –2.67) (t=11.78, df=5, p<0.001).
Results from the separate Affect Grid study confirmed the capacity of picture-caption pairs to elicit feelings as intended. In experiment 1, the mean pleasantness rating of negative picture-caption pairs was 2.76, and the mean rating of reference picture-caption pairs was 3.92 (t=4.08, df=7, p<0.01). In experiment 2, the mean pleasantness rating of positive picture-caption pairs was 7.38; for control picture-caption pairs, the mean rating was 6.49 (t=4.53, df=7, p<0.01). In experiment 3, the mean pleasantness rating of positive picture-caption pairs was 7.82, and that of negative picture-caption pairs was 2.32 (t=19.37, df=7, p<0.001).
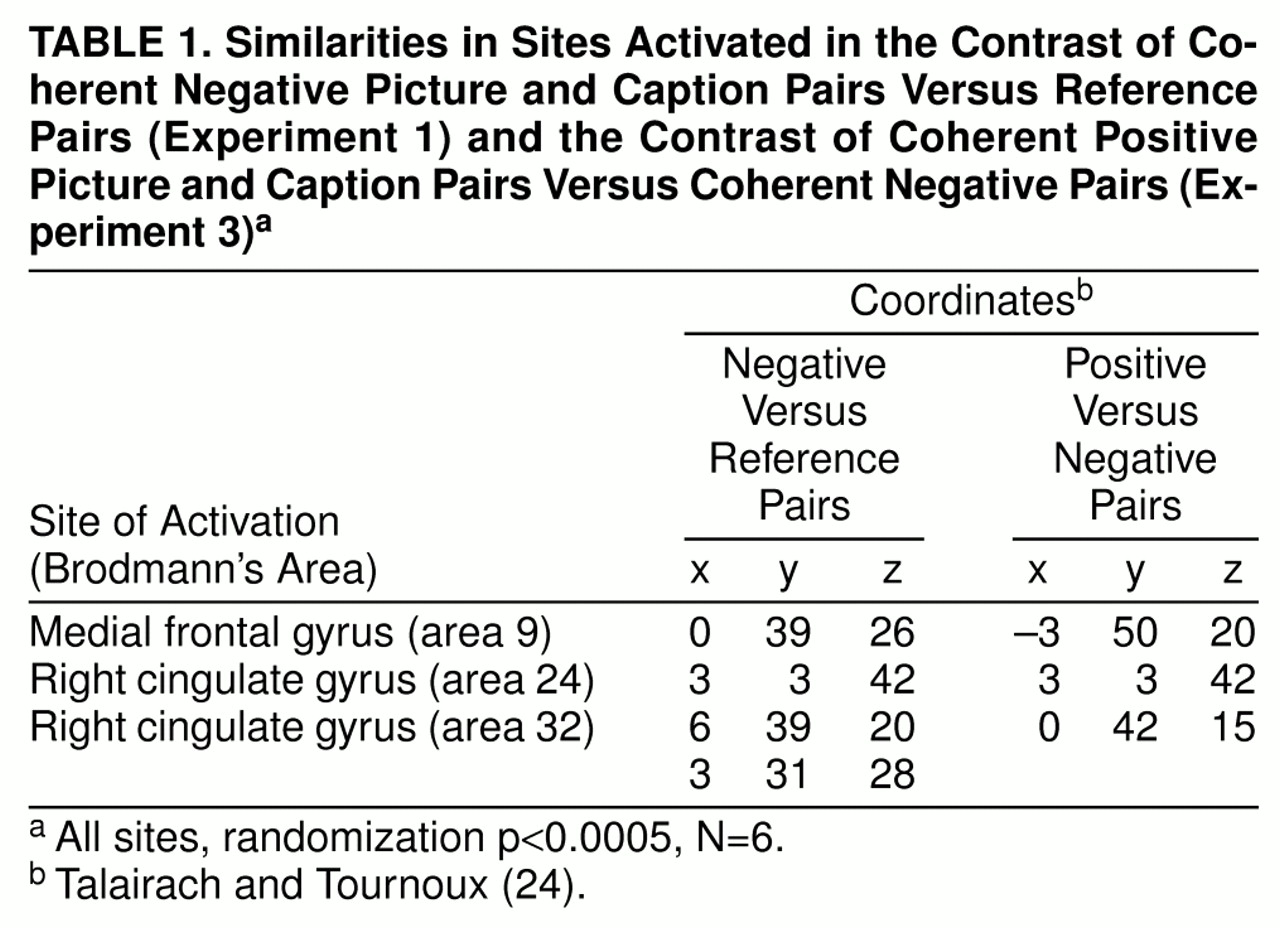
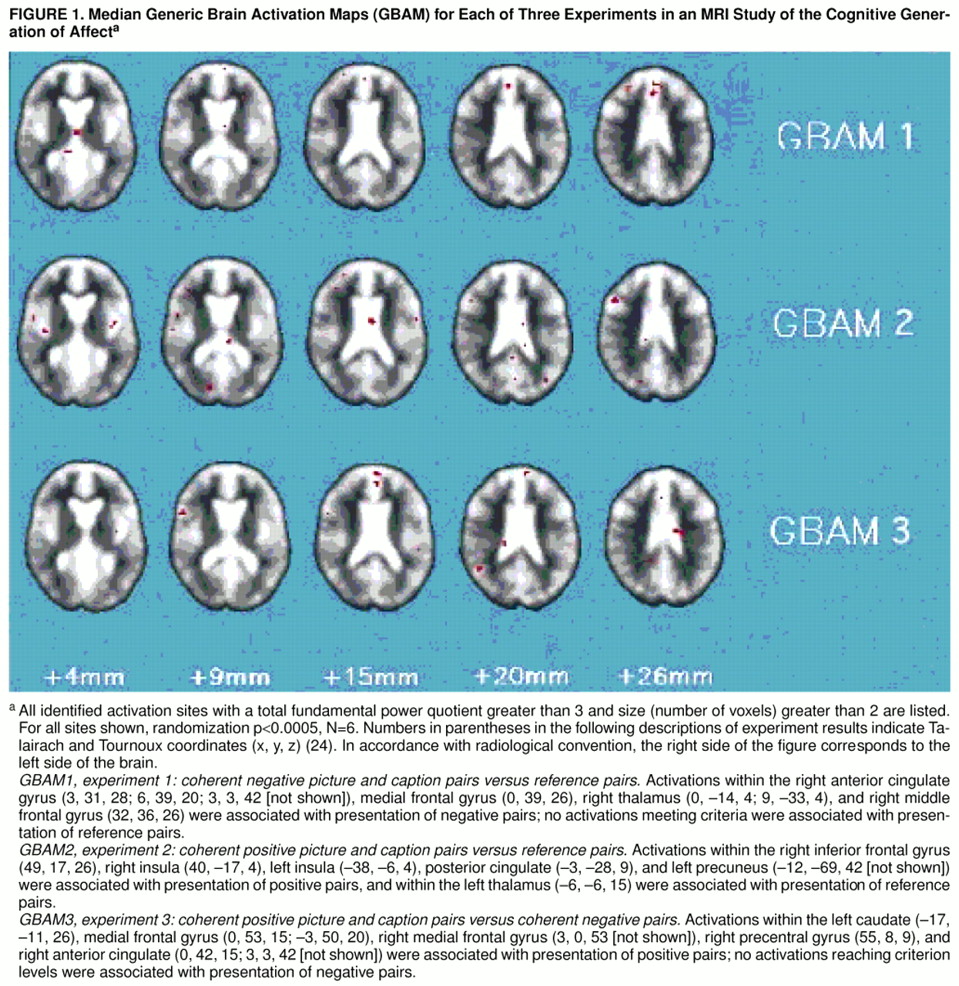
Experiment 1: comparison of brain activation in response to negative and to reference picture-caption pairs. Voxels showing significant brain activation (randomization p<0.0005, N=6) were identified (figure 1). Presentation of negative picture-caption pairs was associated with activation of the medial and right middle frontal gyri (Brodmann’s area 9), right anterior cingulate gyrus (areas 24 and 32), and right thalamus. Presentation of irrelevant pairs in the reference phase did not activate any areas to the designated criterion level.
Experiment 2: comparison of brain activation in response to positive and to reference picture-caption pairs. Voxels showing significant brain activation (randomization p<0.0005, N=6) were identified (
figure 1). Presentation of positive picture-caption pairs was associated with activation of the left and right insula, right inferior frontal gyrus, left splenium, and left precuneus (Brodmann’s area 7). Presentation of irrelevant pairs in the reference phase activated only one brain area (left thalamus) to the designated criterion level.
Experiment 3: comparison of brain activation in response to positive and to negative picture-caption pairs. Voxels showing significant brain activation (randomization p<0.0005, N=6) were identified (
figure 1). Presentation of coherent positive picture-caption pairs was associated with significant areas of activation within the right and left medial frontal gyri (Brodmann’s areas 9 and 6), right precentral gyrus (area 6), right anterior cingulate (areas 24 and 32), and left caudate. None of the areas of activation associated with presentation of negative picture-caption pairs reached the designated criterion level of activation.
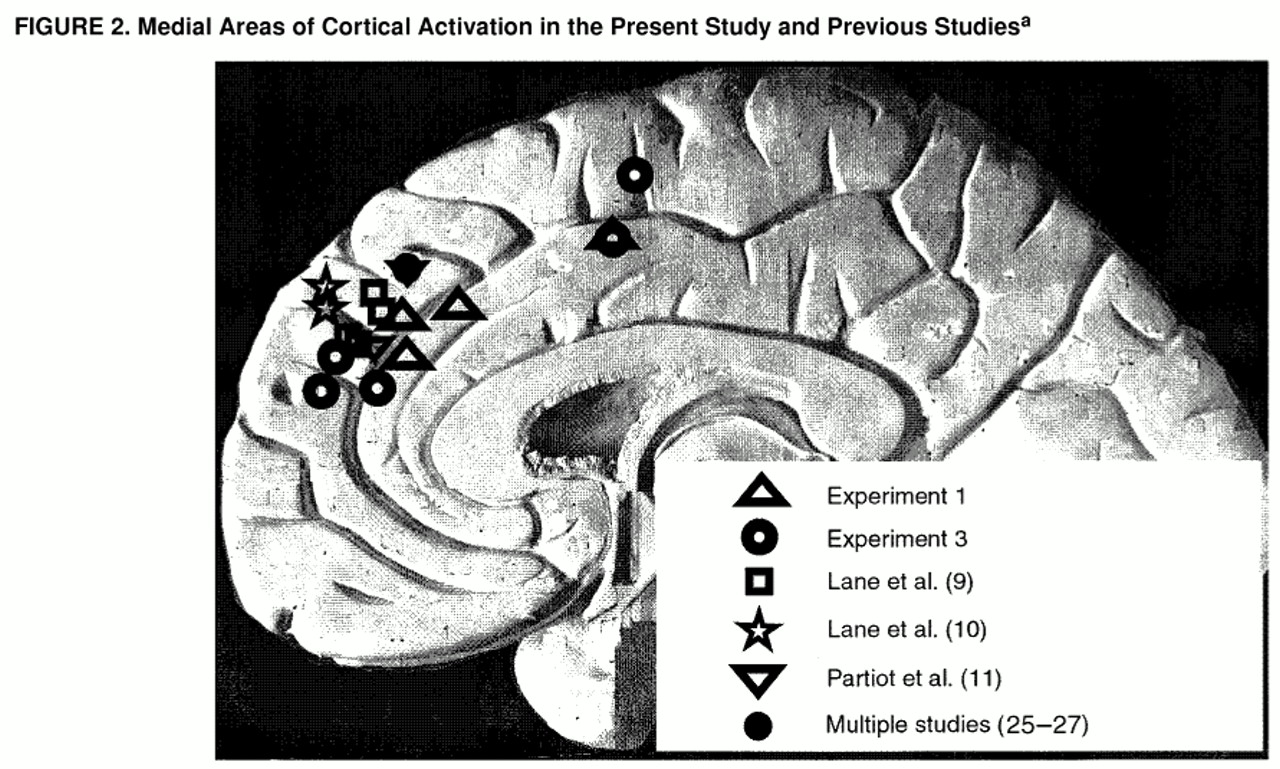
Comparison of experiments 1 and 3. Table 1 and
figure 2 show sites that were activated in both experiment 1 (contrast between coherent negative picture-caption pairs and reference picture-caption pairs) and experiment 3 (contrast between coherent positive picture-caption pairs and coherent negative picture-caption pairs). In both experiments, networks involving similar areas in the medial frontal gyrus (Brodmann’s area 9) and right anterior cingulate gyrus (areas 24 and 32) were activated.
DISCUSSION
The results of experiments 1 and 3 present an interpretable picture very consistent with results from closely related PET studies (
9, 10,
28). Medial prefrontal activations within Brodmann’s area 9 (medial frontal gyrus) and activations within Brodmann’s areas 24 and 32 (right anterior cingulate gyrus) were associated with affective contrasts involving both negative versus reference picture-caption pairs (experiment 1) and positive versus negative picture-caption pairs (experiment 3). These activations were predominantly right-sided, although making confident judgments about lateralization close to the midline is problematic.
The PET studies most closely similar to the present investigation (
9,
10) found emotion-related activation in areas of the medial prefrontal cortex (Brodmann’s area 9, medial frontal gyrus) remarkably similar to those in the present study (figure 2), together with activation of the thalamus (also seen in experiment 1). These studies found similar medial prefrontal activation whether the emotions induced were pleasant or unpleasant and whether the inductions involved presentation of evocative slides, film clips, or recall of memories of emotional events.
The finding that the identified sites in the medial prefrontal cortex (area 9) are activated across a wide range of evoked affects, with the use of different methods of affect elicitation, is consistent with such activation reflecting a central general component of emotion production, rather than more specific processes related to particular cognitive methods of emotion induction or to the orchestration of particular types of emotional response. Processing of higher-order emotion-related meanings plays just such a central general role in the cognitive route of emotion production; we suggest that activation of the areas of the medial prefrontal cortex that we identified is associated with processing of affect-related meanings, either those accessed by currently presented stimuli or, in the case of recall of affective memories, those stored in conjunction with the memories.
Why, if the above hypothesis is correct, did experiment 2 not give evidence of activation of medial prefrontal areas by positive picture-caption pairs? Equally, in experiment 3, why did positive picture-caption pairs produce greater medial prefrontal activation than negative picture-caption pairs if both involved processing of emotion-related meanings?
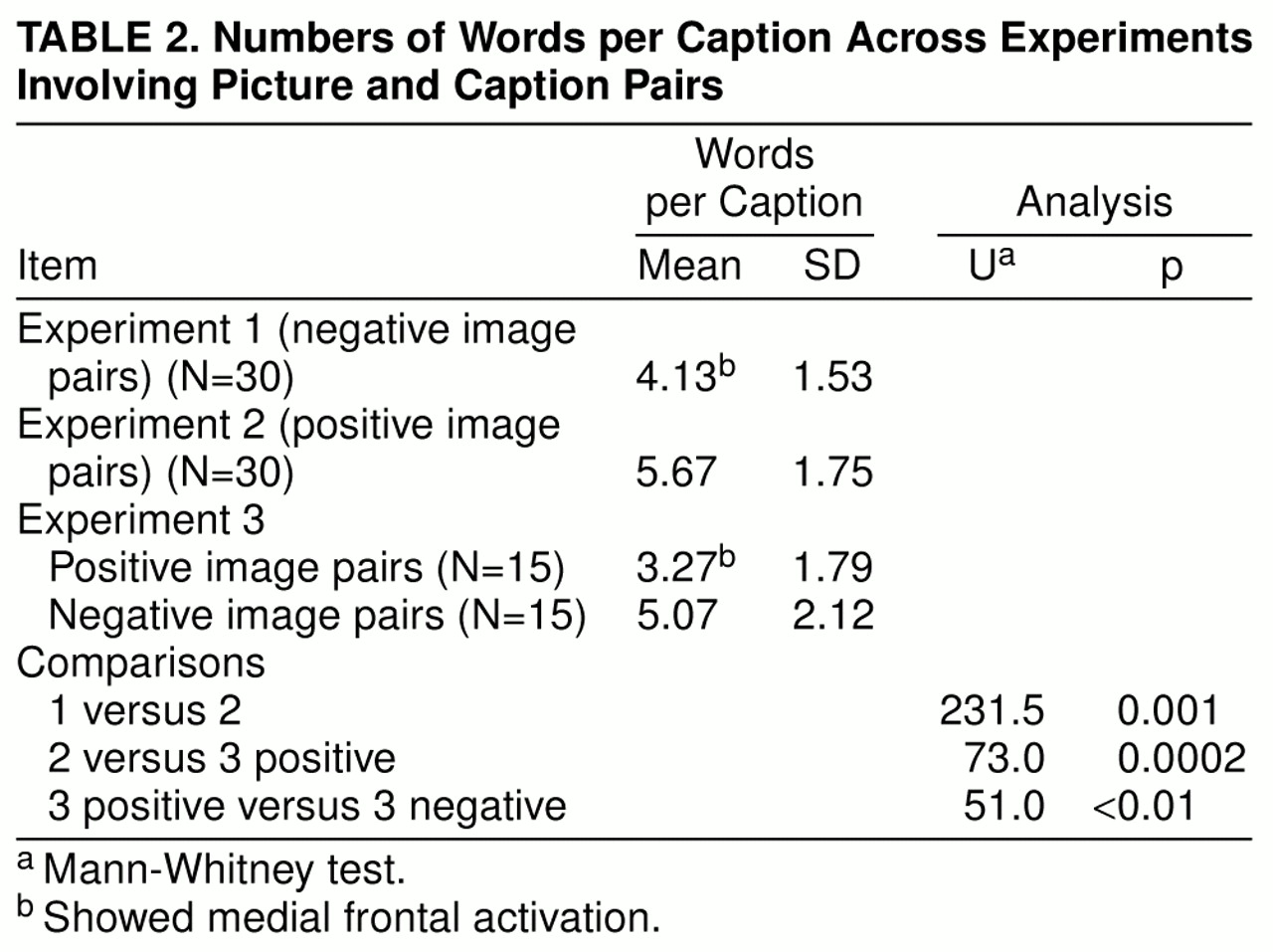
Our best explanation is that activation of the medial prefrontal cortex (area 9) reflected access to preexisting affective meanings, available in memory, and the subsequent processing of those meanings, rather than on-line, effortful synthesis of such meanings. This suggestion is based on analyses of the number of words in the captions in different categories of picture-caption pairs. Within experiments 1 and 2, the number of words in different categories was matched, by design. However, this matching did not preclude the possibility of differences across experiments or within experiment 3.
Table 2 shows that the numbers of words in caption pairs in experiments showing significant medial prefrontal activations were significantly less than in experiments that showed no such activations. We suggest that the number of words in captions reflected the ease with which a given picture-caption pair automatically accessed preexisting emotion-related schematic meanings: brief captions characterized pairs that achieved access relatively instantly and effortlessly (e.g., a brightly colored picture of a cheerful-looking yellow plastic duck, paired with the caption “Hi!”), whereas longer captions characterized pairs that required more extended processing to create affect-related meanings (e.g., a picture of a man painting a cottage, with the caption “Kindness—painting his elderly neighbor’s house”). This suggestion offers a potentially testable post hoc explanation for the observed anomalous results.
This study, using an essentially cognitive method of emotion elicitation, found no evidence of amygdala activation. It is unlikely that this reflected technical limitations of the method of neuroimaging that we used; previous studies (
29), using the same methods of neuroimaging and analysis, have demonstrated such activations in response to more directly perceptual emotive stimuli. Rather, our results support the suggestion of Reiman et al. (
28, p. 923) that although the amygdala, hippocampal formation, and hypothalamus may be involved in emotional response to stimuli that are emotive at a directly perceptual level, they may be less relevant to cognitively elicited emotions.
Consistent with the suggestion that areas of the medial frontal gyrus are involved in the cognitive processing of affective meaning, a recent PET study of word processing (
30) found widespread activation of the left medial frontal gyrus in response to emotional words, together with activation of the orbital prefrontal cortex.
Damage to areas of the medial prefrontal cortex has marked emotional consequences consistent with involvement of these areas in affect-related processing. Patients with lesions of the medial orbitofrontal cortex show disrupted social and emotional behavior and impairments in real-life decision making in spite of otherwise preserved intellectual abilities (
31). Such deficits are consistent with this brain region playing a central role in assessing the affective significance of events and outcomes. Similarly, Rolls (
32) has suggested, on the basis of studies in both monkeys and humans, that decoding and rapidly readjusting the reinforcement value of stimuli is an important function of the orbitofrontal cortex. Such decoding is directly analogous to accessing the affect-related meanings of stimuli.
The activations in Brodmann’s area 9 in this study and in others (
9–
11) are actually somewhat dorsal to the areas of the orbitofrontal cortex damaged in patients studied by Bechara et al. (
31) and noted as consistent areas of activation in PET studies of induced sadness (
6,
7). They are close to areas of the medial prefrontal cortex activated in studies that have involved either “theory of mind” tasks (
25,
26) or the generation of stimulus-independent thought (daydreaming) (
27) (
figure 2). Such tasks have been described as entailing “thinking which is decoupled from stimuli in the immediate environment” (
27, p. 2095). These tasks could also be characterized as requiring access to preexisting representations of schematic mental models, related to self and others, from memory.
It is possible that areas of the medial prefrontal cortex are involved in accessing both affective and nonaffective schematic mental models of personal and social relevance. The present study implicates the medial prefrontal cortex in processing the affect-related schematic mental models that, according to the interacting cognitive subsystems account (
15,
16), encode the affective meanings which generate emotion by the cognitive route.