Autism is a neurodevelopmental disorder that begins in infancy and is characterized by three distinct symptom dimensions: social deficits, speech and communication impairment, and repetitive behaviors and restricted interests (DSM-IV). The etiology, pathophysiology, and genetic transmission of autism are not known, but autism may be best understood as a heterogeneous disorder resulting from multiple genetic and environmental factors, often complicated by neurologic, cytogenetic, neurotransmitter, and immunologic abnormalities.
Recent studies of immunologic aspects of autism suggest that the disorder may have an autoimmune basis in a subset of patients. We previously described
(1) how the disorder shares features of autoimmune diseases, including genetic susceptibility, association with viral infection, and immunologic dysfunction. A link between autism and autoimmune illnesses, such as rheumatoid arthritis, was first described in 1971
(2). Since then, investigators have described cellular and humoral dysfunction and abnormalities associated with complement and the major histocompatibility complex gene expression. Immune abnormalities include evidence of T cell activation, abnormality in the numbers and proportions of T cell subsets, abnormal B and T cell function in vitro, poor antibody production, low levels of IgG and IgA subclasses, abnormal cytokine levels in the sera, and a high frequency of the C4B null allele
(1,
3–
6). Clear evidence of autoimmunity is also present, since antibrain and antimyelin antibodies are found at high frequencies in the sera of autistic children
(7,
8). In a preliminary study of autistic children who received intravenous immunoglobulin treatment, Gupta et al.
(9) demonstrated immune system abnormalities at baseline and improvement in communication and behavioral functions following treatment. However, this study did not use adequate behavioral assessment measures and requires replication in a controlled trial.
The monoclonal antibody D8/17 reacts with an antigen on at least 20% of the peripheral blood B cells of all rheumatic fever patients. In comparison subjects, the D8/17-positive B cells range from 0% to 7%
(10). First-degree relatives of rheumatic fever patients demonstrate an intermediate percentage of D8/17-positive B cells. Expression of the D8/17 marker is low in patients with rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus and in those with poststreptococcal glomerulonephritis, suggesting that D8/17 expression on a B cell is not solely the result of chronic autoimmune stimulation of B cell activation by an undefined streptococcal antigen. The function of this antigen and its role in the pathophysiology of rheumatic fever have not yet been established
(11), although in Sydenham’s chorea, which occurs in 10% of rheumatic fever patients, antibodies to group A β-hemolytic streptococcus cross-react with neuronal cells and cause repetitive behaviors.
Autoimmune findings have been demonstrated in obsessive-compulsive disorder (OCD) as well. Cross-sectional and longitudinal studies support a relationship among group A β-hemolytic streptococcal infections, positive antineuronal antibody titers, and neuropsychiatric dysfunction in a subgroup of children with OCD, tics, and Tourette’s syndrome
(12). Swedo et al.
(13) and Murphy et al.
(14) determined the frequency of D8/17 positivity in patients with childhood OCD and Tourette’s syndrome by using an immunofluorescence assay. Both groups found significantly more peripheral B cells expressing D8/17 in the OCD patients than in healthy comparison subjects. These results suggest that D8/17 may serve as a marker for susceptibility among some forms of childhood-onset OCD and Tourette’s syndrome. However, additional research is needed to determine the full impact of this finding in OCD. We have suggested
(15) that if D8/17 levels are found to be correlated with repetitive behaviors as measured by the severity rating on the Yale-Brown Obsessive Compulsive Scale, there might be support for a dimensional approach to D8/17 mediation of compulsive symptoms and repetitive behaviors across traditional diagnostic boundaries.
In the current study, we hypothesized that D8/17 might serve as a marker for susceptibility to autism, especially for the repetitive behaviors present in autistic patients. Thus, our goals were 1) to compare a cohort of autistic subjects with comparable medically ill subjects on the prevalence of dichotomized D8/17 expression; 2) to correlate D8/17 expression with severity of repetitive behaviors in the autistic subjects; and 3) to compare the D8/17-positive and D8/17-negative autistic subjects with regard to severity of repetitive behaviors.
METHOD
Eighteen children with autism (14 boys, four girls) ranging in age from 4 to 13 years (mean=6.9, SD=3.1) completed the protocol. They met the DSM-IV criteria for autistic disorder and were free of major comorbid psychiatric disorders. The DSM-IV diagnosis of autism for each of the 18 patients was made by semistructured interview by a psychiatrist who specializes in autism research (E.H. or C.C.). In addition, for nine of the subjects the diagnosis was confirmed by the Autism Diagnostic Interview
(16), with complete agreement between the DSM-IV and Autism Diagnostic Interview diagnoses. The subjects’ full-scale IQs ranged from 52 to 109 (mean=80, SD=20). Fourteen medically ill children (six boys, eight girls) ranging in age from 1 to 18 (mean=6.0, SD=4.6), who were comparable on socioeconomic, racial, and geographic factors, were used as comparison subjects. The illnesses in this group included surgery for small bowel resection, cardiac catheterization, asthma, sepsis, Kawasaki’s disease, and polycystic kidney disease complicated by hypertension.
The Yale-Brown Obsessive Compulsive Scale
(17), a clinician-administered 10-item questionnaire that uses 5-point scales to rate time spent, interference, distress, resistance, and control, was administered to assess the severity of repetitive thoughts and behaviors. It is a reliable and valid scale for severity of obsessions and compulsions, and the compulsion subscale reliably assesses compulsive and repetitive behaviors in autistic subjects and is sensitive to change in treatment studies
(18). All patients and their parents gave written informed assent and consent, respectively, for participation in this study.
Heparinized blood for immunofluorescent studies was collected from the autistic subjects and comparison subjects on the same day and stored at room temperature for no more than 24 hours before the assay. Monoclonal antibody D8/17 is a mouse monoclonal IgM antibody originally prepared from fusions of spleen cells from mice repeatedly immunized with isolated B cells obtained from patients with rheumatic fever or rheumatic heart disease. The monoclonal antibody D8/17 was produced in the Laboratory of Clinical Microbiology and Immunology at Rockefeller University, and the immunofluorescent assay was run as described elsewhere
(11,
19). In brief, by means of an indirect immunofluorescence assay, B cell staining was determined on fresh mononuclear cell preparations. The peripheral blood B cells were then stained with monoclonal antibody D8/17 and fluoresceinated antimouse IgM and counted with a fluorescent microscope. B cells were identified by using concomitant staining with phycoerythrin-conjugated monoclonal anti-DR human leukocyte antigen class II reagents. The results were expressed as the percentage of positive cells among 500 counted. All of the readings were done by an examiner blinded to subject status, and they were checked by another examiner for accuracy. On the basis of previous studies, a D8/17-positive case was defined as a value of one standard deviation above historical comparison values, i.e., ≥11% antigenic expression. The normal comparison mean values range from 0% to 7%
(10). To assess for recent streptococcal infection, blood samples were collected and assayed for streptococcal antibodies (antistreptolysin-O and antiDNase B).
The results of the immunofluorescence assays were classified as negative (0%–10% D8/17 expression) or positive (≥11%). All tests of significance used the 0.05 level of significance and were two-sided. For goal 1, the proportion of positive expression, out of positive plus negative, was the estimate of the occurrence, and Pearson’s chi-square test was applied. For goal 2, the autistic subjects’ D8/17 assay value was correlated with their Yale-Brown Obsessive Compulsive Scale compulsion score by means of Pearson correlation. For goal 3, we compared the compulsion scores of the D8/17-negative and -positive autistic subjects, conservatively using the less significant of the pooled and separate variance estimate t tests.
RESULTS
The autistic and medically ill groups did not significantly differ in age (t=0.62, df=22, p=0.54) or sex distribution (p=0.07, Fisher’s exact test), and age did not significantly influence D8/17 level (r=0.23, df=16, p=0.35). Fourteen (77.8%) of the 18 autism subjects were positive for the D8/17 marker (≥11%) and four (22.2%) were negative (<11%). In contrast, three (21.4%) of the 14 comparison subjects were positive and 11 (78.6%) were negative for the marker. Thus, the autistic subjects were significantly more likely than the comparison subjects to be classified as D8/17 positive (Pearson χ2=10.04, df=1, p=0.002).
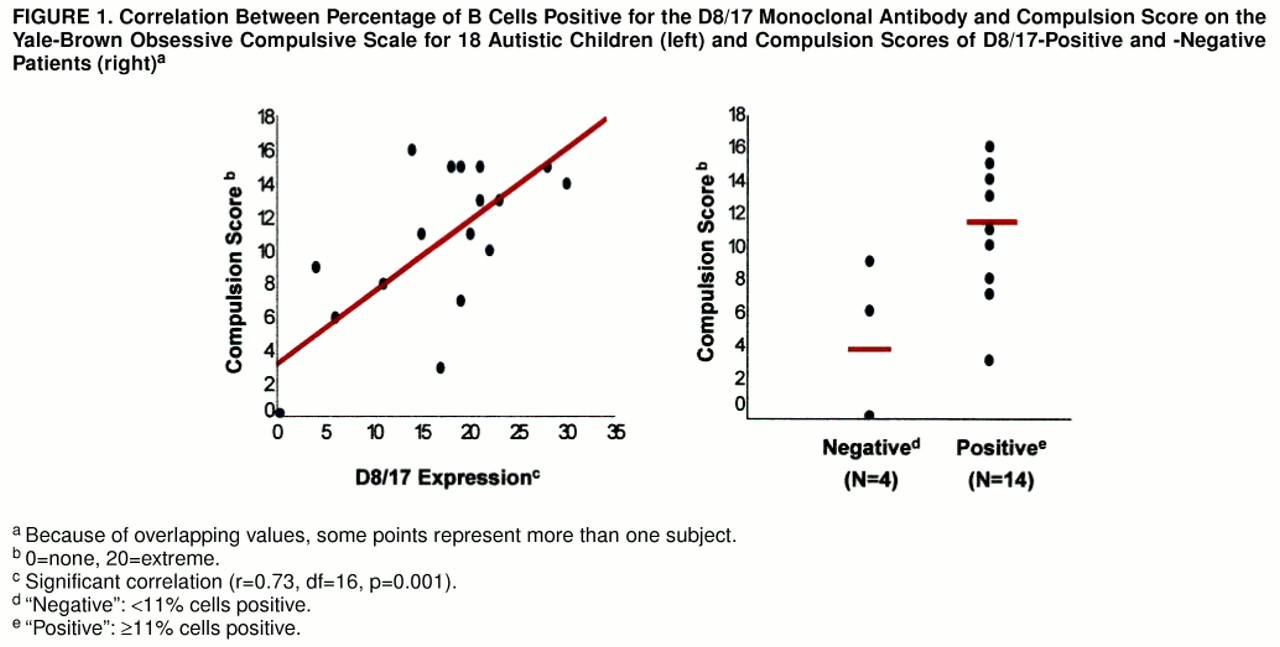
Of interest, the severity of repetitive behaviors among the autistic children, as measured by the compulsion subscale of the Yale-Brown Obsessive Compulsive Scale, significantly positively correlated with D8/17 values (r=0.73, df=16, p=0.001), such that the autistic patients with greater compulsive/repetitive behavior had greater D8/17 antigen positivity (figure 1, left side). In contrast, there was no significant (or nearly significant) correlation between D8/17 value and score on the Autism Diagnostic Interview social (r=0.01) or communication (r=0.29) algorithm. When a dichotomous approach was used it was seen that the D8/17-positive autistic patients had a significantly higher mean Yale-Brown Obsessive Compulsive Scale compulsion score (mean=11.9, SD=3.8) than did the D8/17-negative patients (mean=3.7, SD=4.5) (t=3.29, df=4.29, p=0.03) (figure 1, right side). Thus, D8/17 positivity was associated with greater compulsive severity in children with autism, and the D8/17-positive patients had greater compulsive severity.
Only one (5.6%) of the 18 autistic subjects had a high antistreptolysin-O titer (≥200 IU/ml), and only four (22.2%) had high levels of antiDNase B (≥1:64 for preschool and ≥1:170 for school-age children; smaller ratio equals greater dilutional titer, which corresponds to higher antistreptococcal antibody level), suggesting that the majority of the autistic children had had no recent streptococcal exposure. The study subjects and their parents did not report any history of rheumatic fever.
DISCUSSION
The cause of autism is not known, although there is evidence of immunologic dysfunction in the disorder
(1, 3–8). Monoclonal antibody D8/17 identifies a B cell antigen that denotes susceptibility to rheumatic fever
(10). Recently, high levels of D8/17 have been documented in a subgroup of OCD patients
(13, 14).
Our data demonstrate significantly greater expression of monoclonal antibody D8/17 in a subgroup of autistic children than in matched medically ill children. D8/17 has been proposed as a trait, rather than state, marker of rheumatic fever, and thus it has been suggested that D8/17 should be treated as a dichotomous variable (i.e., positive or negative)
(20). However, the percentage of B cells positive for D8/17 may fluctuate with disease exacerbation (J.B. Zabriskie, personal communication), and severity of repetitive behavior in autism may fluctuate in response to antiobsessional treatment with serotonin reuptake inhibitors
(21), suggesting that a continuous or dimensional approach to studying their relationship may be justified. Severity of repetitive behaviors as assessed by the compulsions score on the Yale-Brown Obsessive Compulsive Scale strongly positively correlated with D8/17 expression. Alternatively, in a dichotomous approach, the D8/17-positive patients had significantly higher scores for repetitive behavior than did the D8/17-negative patients.
D8/17 antigen expression may represent a genetic vulnerability to autism and an environmental susceptibility to autism. The nature of the association between D8/17 positivity in autism and abnormal immune response to group A β-hemolytic streptococcal is unclear at this time. A major manifestation of rheumatic fever is Sydenham’s chorea, in which antibodies to β-hemolytic streptococcal cross-react with neuronal cells and cause motoric and behavioral abnormalities, including obsessive-compulsive symptoms
(13, 14), and autoantibodies directed against the caudate and subthalamic nuclei have been identified
(22). D8/17 expression and red cell Na
+/H
+ antiporter activity were positively correlated in rheumatic fever patients in one study
(23), and it was suggested that D8/17 may be associated with cation transport in cell membranes
(23), not just of erythrocytes but perhaps of brain cells as well
(24).
Our preliminary data suggest that the B cell alloantigen identified by the monoclonal antibody D8/17 may identify a subgroup of autistic subjects. Future research should examine whether antineuronal antibodies are involved in the pathogenesis of autism and should compare D8/17-positive and D8/17-negative autistic subjects for the presence of antineuronal antibodies, differences in familial transmission, and differential response to specific therapeutic strategies.