The development of posttraumatic stress disorder (PTSD) is intrinsically related to the experience of overwhelming trauma. Because corticotropin-releasing hormone (CRH) is one of the principal central nervous system (CNS) effectors of the organismic response to stress, the regulation of this neurohormone in PTSD has been of interest, and CNS CRH hypersecretion has been hypothesized
(1). Indeed, preliminary results from a study using lumbar puncture indicate that CSF CRH concentrations are elevated in combat veterans with PTSD
(2). We sought to more closely examine the hypothesis that CRH is hypersecreted into the CNS of patients with PTSD by using a technique of serial CSF sampling in which CSF aliquots were collected over a 6-hour period by means of an indwelling, flexible subarachnoid catheter.
Use of continuous or serial CSF sampling confers several advantages over standard lumbar puncture in the examination of CSF CRH concentrations. First of all, CRH appears to be both secreted into human CSF in a pulsatile manner
(3) and rapidly transported out of the CNS
(4), rendering a single time assessment of its concentration of limited value. In addition, human CSF CRH levels exhibit a diurnal variation in concentration
(5). Moreover, the procedure of lumbar puncture is associated with significant anxiety in most human subjects. We have shown that this procedure is associated with acute increases in levels of CSF CRH
(6). To control for these problems, we have used serial CSF sampling in combat veterans with PTSD and normal comparison subjects in order to determine basal levels of CRH.
RESULTS
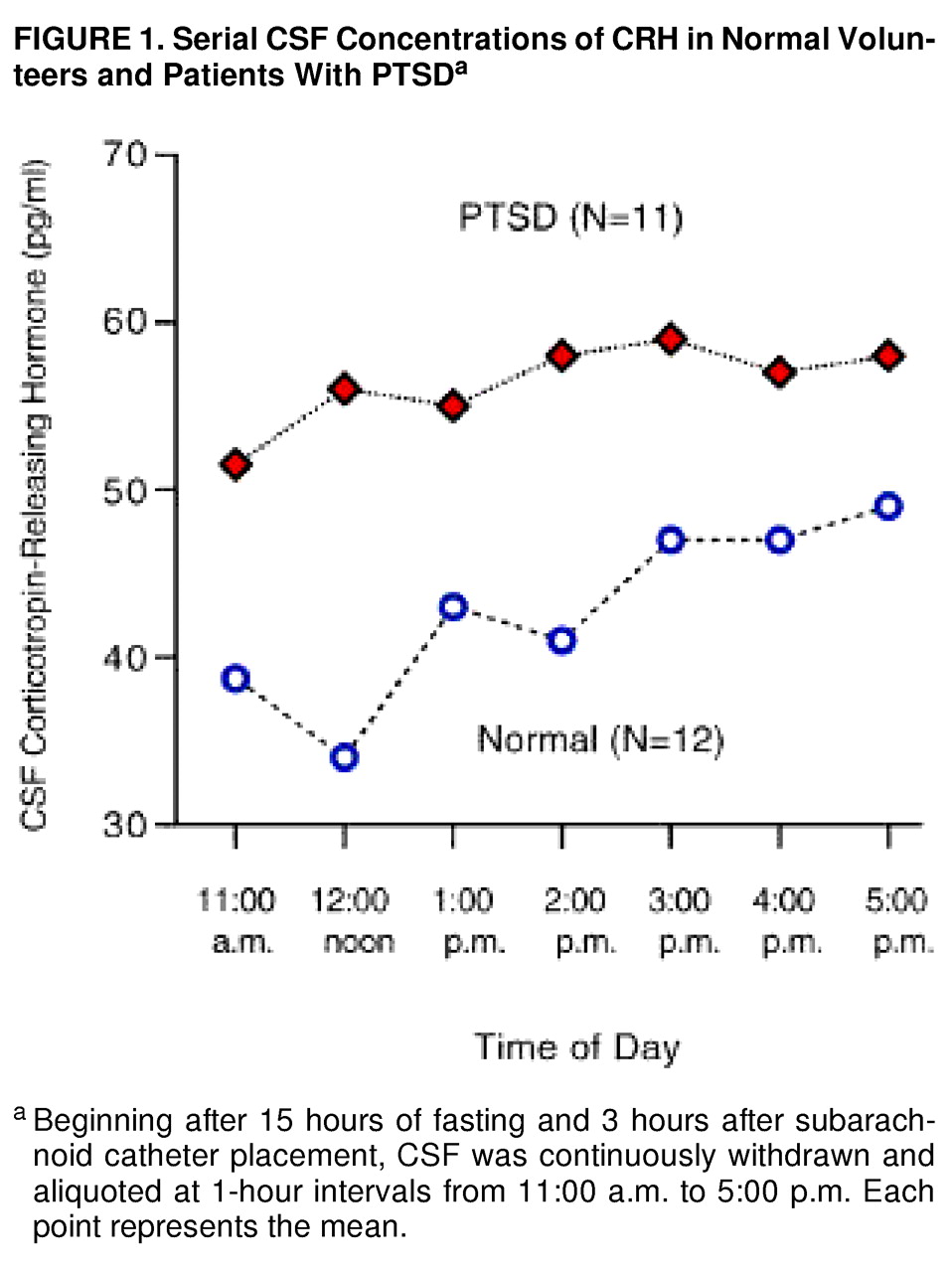
By using a three-way repeated measure ANOVA (diagnostic group by smoking status by CRH concentrations at seven time points), we found no interaction effects. There were two statistically significant main effects. CSF CRH concentrations were significantly higher in the patients with PTSD than in the normal volunteers (55.2 pg/ml, SD=16.4, versus 42.3, SD=15.6) (F=4.74, df=1, 16, p<0.04). Mean CSF CRH concentrations were lower in nonsmokers than in smokers (43.7 pg/ml, SD=13.0, versus 52.1, SD=19.2), but the difference was not significant (F=–1.12, df=1, 16, p<0.31).
In addition, there was a significant CSF CRH time main effect (
figure 1), which remained significant after the Huynh-Feldt correction was used (F=2.39, df=5.23, 83.67, p<0.05). Three subjects, one comparison subject and two patients, were not included in the ANOVA because of missing time point concentrations. Conclusions did not change when all 23 subjects were used in a simple two-way factorial (diagnosis by smoking) in which concentration responses were averaged over time.
No significant difference in 24-hour urinary-free cortisol excretion was evident between normal volunteers and patients with PTSD. Mean urinary-free cortisol excretion was 76.2 µg/24 hours (SD=19.7) in normal subjects and 84.4 µg/24 hours (SD=55.3) in patients with PTSD (t=–0.40, df=17). Although the mean 24-hour urinary-free cortisol excretion was higher in the smokers (97.5 pg/ml, SD=57.3) than the nonsmokers (67.4 pg/ml, SD=19.7), the difference was not significant (t=–1.56, df=17).
No significant relationship between mean CSF CRH concentrations and mean 24-hour urinary-free cortisol excretion was observed (r=–0.08, df=17).
There was no significant correlation found between mean CSF CRH concentrations and overall PTSD severity, as measured by total scores on the Clinician-Administered PTSD Scale (r=–0.04, df=10), in the PTSD patients. Similarly, no significant correlations were observed between CSF CRH and the intrusion (r=–0.35, df=10), avoidance (r=–0.08, df=10), or hyperarousal (r=0.39, df=9) subscales of the Clinician-Administered PTSD Scale. The correlation between CSF CRH concentrations and depression, as measured by the Hamilton Depression Rating Scale, was positive but not significant (r=0.35, df=10). However, with removal of an outlier (a dysthymic patient with a Hamilton depression score of 22), a significant positive correlation was observed (r=0.63, df=9, p=0.05).
The correlation between 24-hour urinary-free cortisol excretion and PTSD symptoms, as measured by the total score on the Clinician-Administered PTSD Scale, was negative and significant (r=–0.72, df=9, p<0.02). Correlations between the 24-hour excretion of urinary-free cortisol and subscale scores on the Clinician-Administered PTSD Scale were negative and significant for the intrusion (r=–0.66, df=9, p<0.05) and hyperarousal (r=–0.65, df=9, p<0.05) subscales and were negative and approaching significance for the avoidance subscale (r=–0.55, df=9, p=0.10). There was no significant correlation between 24-hour urinary-free cortisol and Hamilton depression scores (r=–0.10, df=9).
DISCUSSION
We obtained serial CSF samples over 6 hours and observed higher CSF CRH concentrations in combat veterans with PTSD than in healthy subjects. None of our patients was depressed at the time of the study, all had been abstinent from drugs of abuse and alcohol for at least 6 months before the study, and six of the 11 had never abused drugs or alcohol. That CSF CRH levels were elevated in our patients with a history of alcoholism is all the more striking because we had previously noted very low CSF CRH levels in abstinent male alcoholics
(9). Thus, our finding supports the recent results of Bremner et al.
(2), who obtained CSF by means of a single morning lumbar puncture and also found elevated CSF CRH levels in a similar cohort of patients with PTSD, and extends them by controlling for the stress of needle insertion and by sampling over a period of 6 hours.
Our past study
(6) suggests that smokers have lower CSF CRH concentrations than nonsmokers after 11–17 hours of abstinence. The data from the present study are generally consistent with these previous findings (although few normal volunteer cigarette smokers were included in the present cohort). It is of interest that carbon monoxide (found in cigarette smoke) has been shown to be a negative regulator of hypothalamic CRH in vitro
(12). Accordingly, direct comparison of nonsmoker normal subjects with nonsmoker PTSD patients was performed. When only nonsmokers were compared, normal subjects had CSF CRH concentrations of only 42.5 pg/ml (SD=15.0), whereas the PTSD patients had CSF CRH concentrations of 67.5 pg/ml (SD=15.1).
CSF CRH concentrations presumably reflect the integrated effects of CRH synthesis and release, enzymatic degradation, and clearance into the plasma from CSF (which is unidirectional in the case of CRH)
(4). We cannot determine the status of these respective influences on CSF CRH levels in our patients and normal subjects from the data at hand.
CRH in the lumbar spinal canal is largely extrahypothalamic in origin, and its concentrations have not shown significant correlations with plasma cortisol concentrations
(5,
6). Therefore, the inability in the present study to discern a significant relationship between mean CSF CRH levels and 24-hour urinary-free cortisol excretion was not surprising.
As a group, our PTSD patients had normal 24-hour urinary-free cortisol excretion, a finding compatible with the normal to low 24-hour urinary-free cortisol levels reported in all previous studies
(13–
16) except one
(17). In addition, we observed a significant negative correlation between 24-hour urinary-free cortisol excretion and PTSD symptoms, a finding consistent with the results of most prior investigations. While Smith et al.
(1) found a positive correlation between 8:00 a.m. plasma cortisol levels and PTSD symptoms, other studies, including those that quantified urinary-free cortisol excretion
(15), 8:00 a.m. plasma cortisol
(16,
18), or salivary cortisol
(19,
20), all demonstrated an inverse correlation between cortisol concentrations and PTSD symptoms.
In summary, using serial CSF sampling, we found high basal CSF CRH concentrations and normal 24-hour urinary-free cortisol excretion in combat veterans with PTSD, a combination of findings that appears to be unique among psychiatric conditions studied to date. It will prove of interest to characterize the responsivity of CSF CRH to provocative challenge (evocation of symptoms) in patients with PTSD.