Research has documented genetic links between schizophrenia and schizotypal personality disorder
(1–
3). Other investigations have demonstrated that subjects with schizotypal personality disorder manifest cognitive impairments
(4), brain abnormalities
(5), and premorbid deficits
(6,
7) similar to those associated with schizophrenia. Notable among the last mentioned are marked increases in interpersonal and thought abnormalities during adolescence
(6).
The features of schizotypal personality disorder parallel the prodromal signs of schizophrenia and have been shown to occur in preadolescents and adolescents
(8–
11). It is assumed that a subgroup of persons with schizotypal personality disorder will progress to schizophrenia. Speculations on the factors that potentiate this progression involve both psychosocial stress and perinatal complications
(12–
16).
This article presents initial results from a study intended to document the development of schizotypal personality disorder and identify predictors of psychotic symptoms. The focus is on adolescence, assuming that the pubertal period is associated with neuromaturational processes that can trigger the expression of latent vulnerabilities.
In the first evaluation, a primary goal was to verify risk status through the assessment of vulnerability markers, particularly physical indicators. Minor physical anomalies are physical characteristics known to be associated with developmental disorders
(17,
18). Patients with schizophrenia have more minor physical anomalies than other psychiatric patients and normal subjects
(19–
22), and minor physical anomalies are related to earlier onset of the illness
(23. Dermatoglyphic abnormalities are also associated with developmental disorders
(24–
27), and differences in right- and left-hand finger ridge counts (“fluctuating dermatoglyphic asymmetries”) are elevated in schizophrenia
(28–
30).
Dysmorphic signs originate during fetal development, primarily during the second trimester
(17,
18,
26,
27). Both genetic and prenatal factors contribute to minor physical anomalies and fluctuating dermatoglyphic asymmetries. We are not aware of any report documenting minor physical anomalies or fluctuating dermatoglyphic asymmetries in schizotypal personality disorder; however, the fact that subjects with schizotypal personality disorder manifest brain abnormalities similar to those observed in schizophrenia
(5) suggests that they would also show morphologic signs of neurodevelopmental deviation. Thus, it was hypothesized that schizotypal personality disorder would be associated with increased minor physical anomalies and fluctuating dermatoglyphic asymmetries.
Like dysmorphic signs, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis can result from prenatal insults
(31,
32). It has been shown that HPA dysregulation is associated with schizophrenia, as well as with mood disorders and some other psychiatric syndromes
(33,
34). It has therefore been suggested that the HPA axis acts as a nonspecific moderating system that can potentiate the expression of a variety of disorders and mediate the effects of stress on symptom expression
(34–
37). A neural mechanism for this effect in schizophrenia is indicated by evidence that cortisol augments mesolimbic dopamine activity and is correlated with symptom severity
(34). It is thus plausible that the HPA axis plays a role in potentiating the prodromal expression in individuals at risk. In this study we tested the hypothesis that schizotypal personality disorder is associated with heightened cortisol release.
METHOD
The study groups were 20 subjects (eight of whom were female) who met the DSM-IV criteria for schizotypal personality disorder, 20 subjects (seven female) who met the DSM-IV criteria for another axis II disorder or conduct disorder (the group with other personality disorders), and 26 subjects (nine female) who did not meet criteria for any axis II disorder (the normal comparison group). At the initial evaluation, no subject met diagnostic criteria for an axis I disorder.
Subjects were recruited from the community by means of announcements directed at parents. The announcements described the diagnostic criteria for schizotypal personality disorder in lay terms and indicated that assessments of children who manifested such problems were being conducted for research purposes at Emory University. (The advertisement stated that the child was eligible if he or she was “experiencing problems in at least two of the following areas: social relationships, unusual ideas or behavior, emotional reactions or fears.”) As expected, this recruitment procedure yielded a large number of responses from parents, and their children showed a broad range of severity and chronicity of behavioral problems. A screening interview was conducted by telephone. It was first determined whether the child met the study exclusion criteria (i.e., no medical or neurologic conditions or mental retardation); then the parent was briefly interviewed about the child’s behavior. In addition to identifying potential subjects with schizotypal personality disorder, the screening also served to identify subjects with other axis II disorders for inclusion in the comparison group. Some respondents described symptoms that suggested disorders other than schizotypal personality disorder, whereas others described normal adolescent behavior. Children who seemed likely to meet criteria for inclusion in one of the diagnostic groups were scheduled for assessment. As required by university research guidelines, parents were informed that they would be provided with feedback on the results of the assessment.
The normal comparison group comprised children who did not meet criteria for an axis I or axis II disorder. Some were drawn from respondents to the announcement (N=20), and others were adolescents whose parents had listed them in the University Research Registry (N=6). (The registry contains names of children whose parents registered them as infants for developmental studies. Only one of the six participants drawn from this registry had participated in any previous research, and that was a study of childhood attitudes.) The fact that most of the normal subjects were recruited in the same manner as the other two groups afforded some control for any biases produced by the recruitment method. No differences between the registry subjects and the other normal comparison subjects in demographic or clinical characteristics were revealed.
The mean age was 14.2 years (SD=1.2) for the schizotypal personality disorder group, 14.7 years (SD=2.2) for the other personality disorders group, and 13.9 years (SD=1.6) for the normal comparison group. Analysis of variance revealed a significant difference among the groups in mean age (F=5.26, df=2, 65, p<0.01), with the normal comparison group being younger than the other groups. There were no group differences in sex or race (schizotypal personality disorder group: 17 Caucasian and three African American subjects; other personality disorders group: 17 Caucasian and three African American; normal comparison group: 23 Caucasian, one Hispanic, and two African American).
The diagnostic evaluation included the following: the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II)
(38) questionnaire and interview, the SCID—Patient Version (SCID-P)
(39) psychotic screen (and the complete SCID-P if indicated), and the Beck Depression Inventory
(40). The Beck inventory was administered to obtain information for ruling out axis I mood disorder. In addition to these measures, information was obtained from parents in order to enhance the validity of the diagnoses. Parents were interviewed and completed a child behavior checklist.
It should be noted that we included subjects with schizoid and paranoid personality disorders in the other personality disorders group, rather than the schizotypal personality disorder group, for the analyses reported here. This decision was based on evidence from several family studies indicating that only the rate of schizotypal personality disorder is elevated in the relatives of probands with schizophrenia
(41). However, we also conducted the data analyses comparing all subjects with cluster A personality disorders with the group that had other personality disorders and the normal comparison group. The results, described below, are consistent with the inferences drawn from the family studies.
In accordance with standard procedures, the SCID-II questionnaires were completed first, and responses were used as a guide for interviews that were conducted by doctoral students in psychology (D.D.W., D.D.) who had had systematic training in administering the SCID. Interviews were videotaped to establish interrater reliability for axis II diagnoses (kappa >0.80). Discrepancies were resolved by consensus.
Consistent with previous reports, there was a high rate of comorbidity, and some subjects with schizotypal personality disorder also met the criteria for another axis II disorder (e g., avoidant, borderline, paranoid personality disorder). Diagnoses in the other personality disorders group were as follows: dependent, N=1; obsessive-compulsive, N=4; paranoid, N=3; schizoid, N=3; borderline, N=1; conduct disorder, N=6; passive-aggressive, N=5; and not otherwise specified, N=2. (The total number of diagnoses exceeds the number of subjects in the other personality disorders group because of comorbidity.) For each subject, ratings for the nine schizotypal personality disorder symptoms on the SCID-II were summed to yield a total schizotypal personality disorder symptom score. Separate positive and negative symptom scores were also derived.
As expected, a number of the participants had taken a psychotropic medication, usually prescribed by a pediatrician in response to parental concerns about the child’s behavior. Of these, 14 had taken medication within the past month: in the schizotypal personality disorder group, five had taken methylphenidate, and two had taken antidepressants; in the other personality disorders group, three had taken methylphenidate, and two had taken antidepressants; in the normal comparison group, two had taken methylphenidate. The rate of stimulant medication in the total study group, 15%, is about double the rate (7%) for this age range in the Atlanta metropolitan area. The prescription of stimulant medication to a subgroup of the schizotypal personality disorder subjects is consistent with research showing substantial attentional problems in both schizotypal personality disorder and schizophrenia
(2,
4).
Evaluations were scheduled to begin at noon. Parents and children were informed that it was necessary for the subjects to avoid the consumption of medications and caffeinated products beginning the day before the evaluation. After complete description of the study to the subjects, written informed consent was obtained from the parents and from the children. The subjects then completed the SCID-II questionnaire and the Beck inventory. Examiners provided assistance when needed. After completion of the questionnaires, the clinical interviews were conducted; then the measures described below were administered.
Minor Physical Anomalies
Morphology of six body regions was examined with the use of the Waldrop and Halverson scale
(18). The following anomalies were scored: head—fine electric hair, hair whorls, large or small head circumference (measured by positioning a tape measure over the points on the forehead and occiput that gave the maximal circumference); eyes—epicanthal fold, hypertelorism; mouth—high-steepled or flat and narrow palate, furrowed tongue, tongue with smooth/rough spots; ears—asymmetrical ears, low-seated ears, adherent earlobes, malformed ears, soft and pliable ears; hand—markedly or slightly curved fifth finger, single transverse palmar crease; feet—third toe longer than or equal to the second, webbed toes, large gap between first and second toes. Each anomaly was scored as present or absent, and a point was assigned for each one scored as present.
Minor physical anomalies were assessed blind to the subject’s psychiatric history and current symptoms. The examiners were two research assistants who underwent systematic training in the administration of the Waldrop and Halverson scale. Training included observation of videotaped examinations, as well as practice evaluations with normal subjects and psychiatric patients. Thirteen subjects received multiple independent assessments of minor physical anomalies. Reliability was determined by means of Cohen’s kappa for pairs of examiners, including the two research assistants and the first author. An overall kappa of 0.68 was obtained.
We used the modified Waldrop and Halverson
(18) scoring criteria described by Green et al.
(19,
20). Published norms, adjusted for age and sex, were used in scoring head circumference
(42) and canthal distance
(43) as being one or two standard deviations beyond the mean. Item scores were summed to yield a total score.
Dermatoglyphics
Handprints were obtained with the use of a colorless ink method
(24). Fingertips were rolled on ink-sensitive paper to produce a clear print.
Three assistants were trained to use the scoring procedures described by Holt
(25). An image enhancer was used to enlarge each print, and the number of dermal ridges was determined for each fingertip by counting ridges touching a straight line between the triradii and the center of the pattern. For fingertips with more than one triradius, the largest count was used. All prints were scored blind to the subject’s diagnostic status. Each fingerprint was scored independently by two research assistants. One of the investigators (D.D.W.) reviewed the counts, and when discrepancies occurred, the print was rescored.
Corresponding digits from the right and left hands were compared for asymmetry. The absolute difference between the numbers of ridges on each pair of digits on the right and left hands was computed, and then these were added together for a total asymmetry score.
For one subject in the schizotypal personality disorder group and one in the other personality disorders group, minor physical anomaly and dermatoglyphic data were not obtained.
Saliva Collection and Cortisol Assay
Plasma, urinary, and salivary cortisol measures are highly intercorrelated, and salivary cortisol appears to be as sensitive a measure of stress reactivity as urinary and plasma cortisol
(44,
45). Further, because saliva sampling is less invasive, it is most appropriate for repeated assessments
(46).
In total, saliva was collected in specimen tubes four times during the assessment, beginning at about 12:30 p.m. and at hourly intervals thereafter. Samples were stored at –20°C. Data on cortisol were unavailable for one subject in the other personality disorders group and one normal comparison subject.
In preparation for assay, samples were rapidly thawed and centrifuged at 300 g for 10 minutes to remove coagulated protein and other insoluble material. Cortisol was assayed in duplicate 200-µl aliquots of the clear supernatant with the use of materials and procedures provided by Incstar Corp. (Stillwater, Minn.). Assays were performed in tubes coated with an antiserum that shows cross-reactivity only with prednisone (83%), 11-deoxycortisol (6.4%), cortisone (3.6%), and corticosterone (2.3%). Standards in the range of 1–30 ng/ml consisted of the serum standards provided with the kit materials diluted with 200 µl of phosphate-buffered saline. Protein concentrations were equalized in standards and samples by adding cortisol-free serum to the samples. For this laboratory, the mean coefficients of variation between duplicates and between assays are typically less than 5%. Compared with the serum standards, the mean recovery of cortisol from saliva has been indistinguishable from 100%.
Statistical Analysis
Nonparametric procedures (Kruskal-Wallis tests, followed by Mann-Whitney U tests) were used for testing hypothesized group differences in minor physical anomalies and fluctuating dermatoglyphic asymmetries. Differences in cortisol levels between diagnostic groups were tested by analysis of covariance (ANCOVA), with age as the covariate. The same analyses were conducted with the inclusion of the other cluster A personality disorders, paranoid and schizoid, in the schizotypal personality disorder group rather than in the other personality disorders group. This was done to determine whether this more broadly defined group yielded similar results. Correlation coefficients were computed to test interrelations among measures, with a one-tailed alpha level of 0.05.
Before analysis, the minor physical anomalies, fluctuating dermatoglyphic asymmetries, and cortisol data were examined for possible outliers. Outliers were identified as being more than 1.5 box lengths from the 75th percentile, where the box length represents the values of 50% of the cases. For the minor physical anomalies, there was one outlier in the normal comparison group. There were three outliers in the normal comparison group for the fluctuating dermatoglyphic asymmetries variable, but no outliers for the cortisol measures. Analyses were conducted excluding outliers for the respective variables. (The analyses yielded the same general pattern of findings when outliers were included.)
RESULTS
Minor physical anomalies.
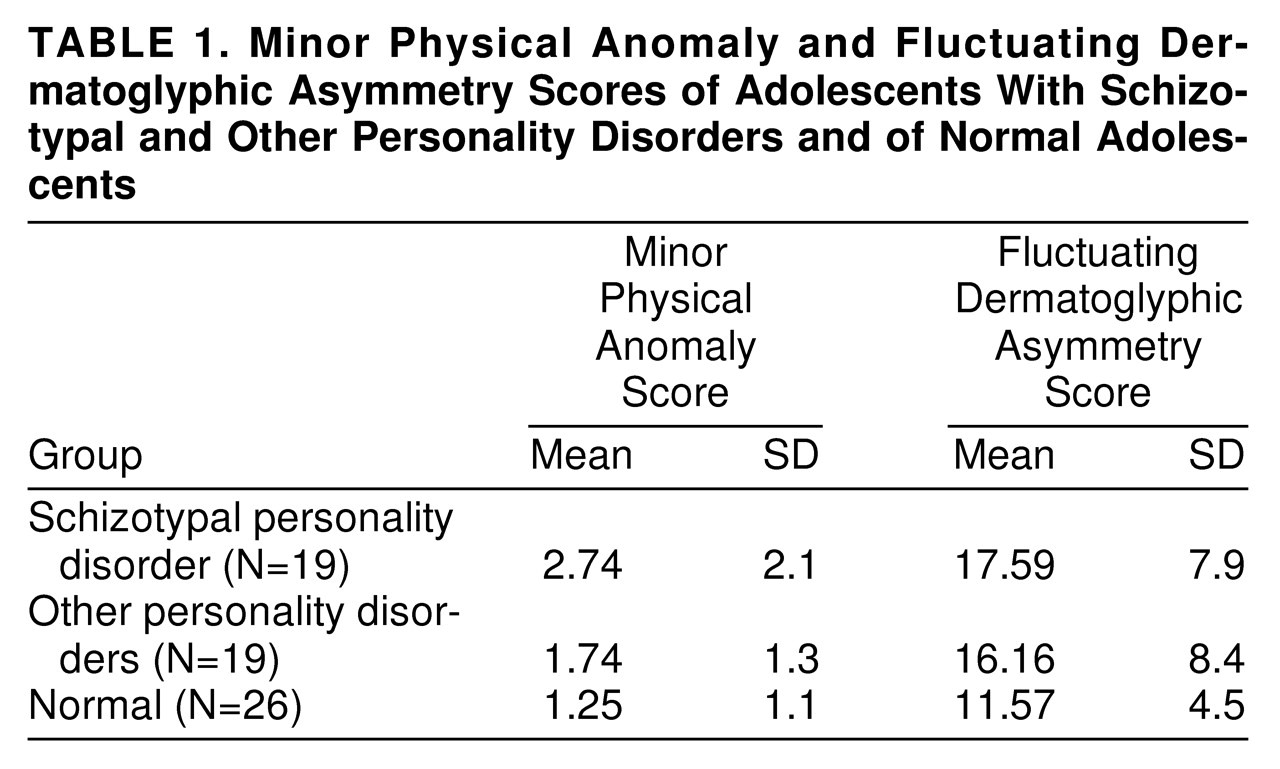
Table 1 presents the group means and standard deviations for minor physical anomalies; there was a significant difference among the groups (Kruskal-Wallis χ
2=5.45, df=2, p<0.05). The subjects with schizotypal personality disorder had significantly higher minor physical anomalies scores when compared with the normal subjects (U=144.5, z=2.26, p<0.02), but in the comparison with the other personality disorders group, the difference did not reach significance (U=133, z=1.43, p=0.07). The other personality disorders group and the normal comparison group were not significantly different. Although there is no standard cutoff for total number of minor physical anomalies, it is noteworthy that 37% (N=7) of the schizotypal personality disorder group, 11% (N=2) of the other personality disorders group, and 4% (N=1) of the normal comparison group exceeded a score of 3. (A similar but attenuated pattern of differences was obtained when the cluster A group was compared with the other personality disorders group and the normal comparison group.)
Fluctuating dermatoglyphic asymmetries.
The means and standard deviations for fluctuating dermatoglyphic asymmetries by diagnostic group are also presented in
table 1; there was a significant overall difference among the groups (Kruskal-Wallis χ
2=2.76, df=2, p<0.05). The schizotypal personality disorder group showed higher scores than the normal comparison group (U=147, z=1.59, p<0.05) but not the other personality disorders group (U=144.5, z=0.54, p=0.58). The subjects with other personality disorders and the normal comparison subjects did not differ. (As with minor physical anomalies, comparison of the cluster A group with the other groups yielded a similar but attenuated pattern of findings.)
For the entire study group, the correlation (Pearson r) between minor physical anomalies and fluctuating dermatoglyphic asymmetries was modest but significant (r=0.23, df=62, p<0.05).
Cortisol.
For each subject, the average of the four cortisol assays was derived.
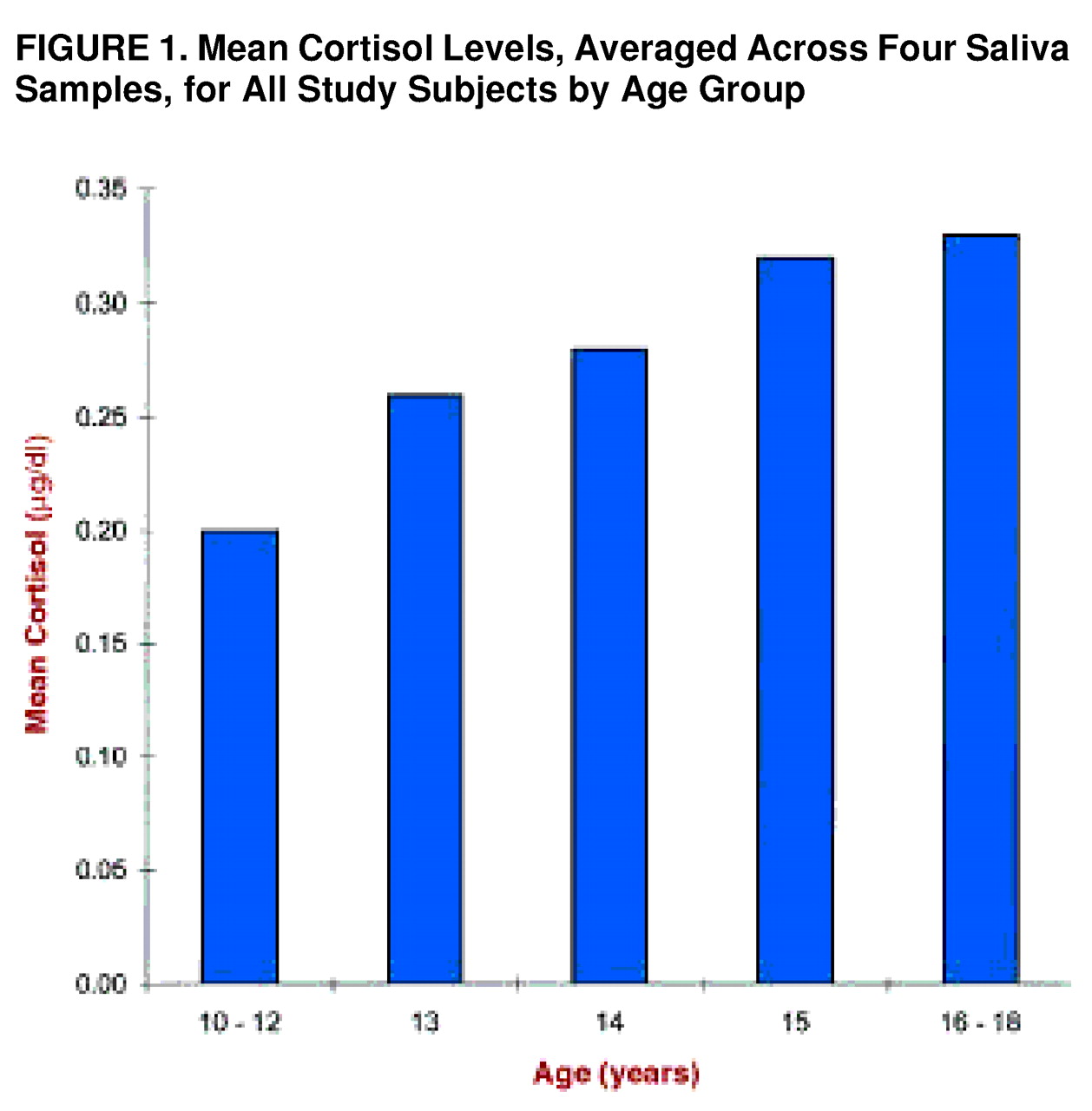
Figure 1 presents the mean values, across groups, by age.
Figure 1 includes data from three subjects who underwent the assessment but were not assigned to a diagnostic group because they were under 12 years of age (two were 10 years old, and one was 11) and may not have comprehended the questionnaires. These subjects are included in
figure 1 to broaden the age range. Also, the 16- to 18-year range is collapsed because there were relatively few subjects at each of these ages (three at 16 years, six at 17 years, and four at 18 years).
Across groups, there was a positive relation between cortisol and age (r=0.48, df=62, p<0.001). When coefficients were computed separately for the groups, the same relationship was observed for the other personality disorders group (r=0.61, df=17, p<0.01) and the normal comparison group (r=0.45, df=23, p<0.05) but not the schizotypal personality disorder group (r=0.14, df=18, p=0.27).
ANCOVA, controlling for age, revealed a significant difference among the groups in mean cortisol level (F=4.19, df=2, 62, p<0.02). The schizotypal personality disorder group had a higher overall mean cortisol concentration than the normal comparison group (F=3.90, df=1, 43, p<0.05) and the other personality disorders group (F=5.00, df=1, 37, p<0.02). For the total study group, mean cortisol level was modestly correlated with total schizotypal personality disorder symptoms (r=0.26, df=62, p=0.01) and negative symptoms (r=0.23, df=62, p=0.03) but not positive symptoms (r=0.15, df=62, p=0.10).
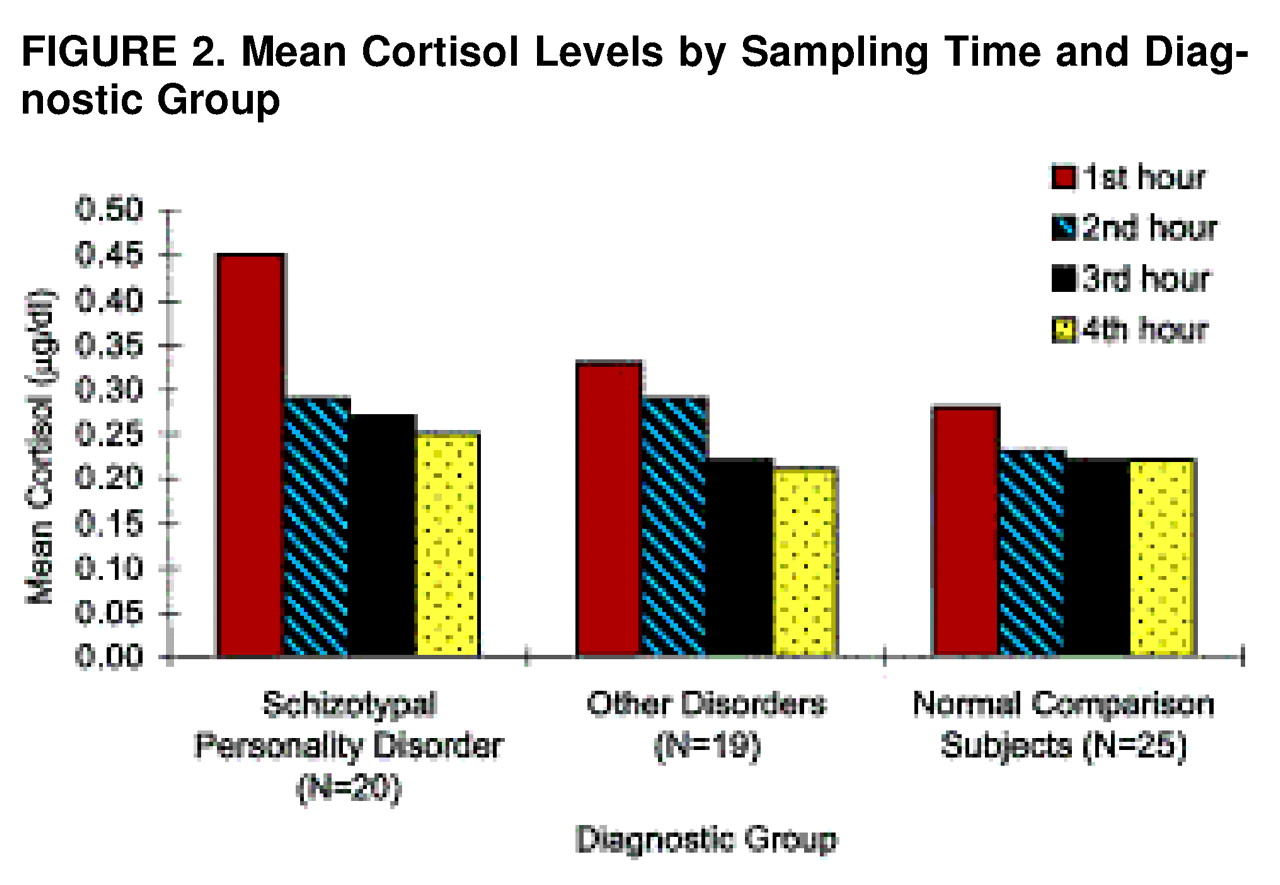
The means for the four cortisol samples are presented separately in
FIGURE 2. All groups showed a decrease in cortisol across time. This reflects the afternoon portion of the diurnal decline. Although the decrease was significant across groups, it appeared more pronounced for the schizotypal personality disorder group.
Group comparisons of the individual cortisol samples, controlled for age, revealed that the schizotypal personality disorder group had a higher time 1 cortisol level than the other personality disorders group (F=3.39, df=1, 37, p=0.04) and the normal comparison group (F=4.79, df=1, 43, p<0.02). Although the schizotypal personality disorder group exceeded the other groups at times 3 and 4, these differences were not significant when age was controlled.
Given that exposure to novelty can heighten cortisol levels (47, 48), the finding of greater group differences in time 1 cortisol concentrations suggests that subjects with schizotypal personality disorder are more responsive to the initial novelty of the assessment. When superimposed on the diurnal change, this results in a greater decline in cortisol values for the schizotypal personality disorder group. We indexed the magnitude of the linear change from the initial level by calculating the slope of the line relating time (time 1 to time 4) to cortisol values for each subject. The mean slope was 0.095 (SD=0.026) for the schizotypal personality disorder group, 0.078 (SD=0.030) for the other personality disorders group, and 0.080 (SD=0.032) for the normal comparison group. A nonparametric test revealed an overall group difference in the slopes (Kruskal-Wallis χ2=5.49, df=2, p<0.05). The subjects with schizotypal personality disorder had a significantly greater linear decline in cortisol release than the normal comparison group (U=157.5, z=2.27, p=0.02), but the difference only approached significance in the comparison with the other personality disorders group (U=131.5, z=1.64, p<0.05). There were no other significant group differences in cortisol slope.
In the entire study group, there was a positive correlation between fluctuating dermatoglyphic asymmetries and cortisol slope (r=0.23, df=58, p<0.05), but the relation between fluctuating dermatoglyphic asymmetries and mean cortisol level only approached significance (r=0.20, df=58, p=0.06). Minor physical anomalies were not correlated with mean cortisol level or cortisol slope.
DISCUSSION
These results provide further support for an etiologic link between schizotypal personality disorder and schizophrenia. The findings indicate that, like schizophrenia, schizotypal personality disorder is associated with an increase in minor physical anomalies and fluctuating dermatoglyphic asymmetries. Assuming that these abnormalities have prenatal origins, a disruption in fetal neurodevelopment may be involved in both disorders.
It also appears, however, that minor physical anomalies and fluctuating dermatoglyphic asymmetries are not specific to schizotypal personality disorder, since there was no significant difference between the schizotypal personality disorder group and the other personality disorders group on these variables. This is consistent with previous reports linking dysmorphic features with a range of mental disorders
(17,
18).
Consistent with prediction, we also found that the subjects with schizotypal personality disorder manifested higher mean cortisol values than both of the other groups. It is noteworthy that the difference between groups was most pronounced when the first sample was obtained. This could reflect group differences in diurnal profile; however, it is also plausible that the schizotypal personality disorder group showed hyperresponsivity to the novelty of the context or procedures
(48).
Our study also replicates the previously reported correlation of salivary cortisol level with age through adolescence
(35). It is of interest that a recent longitudinal investigation of serum cortisol in 28 adolescents found no developmental change
(49). These discrepancies may be due to differences in subjects’ responses to blood versus saliva sampling or may reflect differential subject attrition based on sampling procedure
(50,
51).
If the pubertal period is associated with an enhancement of HPA activity, this suggests a role for the HPA axis as a neuromodulator of the expression of preexisting vulnerabilities during adolescence. When computed separately by group, the correlations between age and cortisol level were significant only for the other personality disorders group and the normal comparison group. This may indicate that the pubertal increase in HPA activity began earlier in the schizotypal personality disorder group. Because these speculations are based on cross-sectional data, confirmation is needed from prospective research.
Studies of nonhuman primates have shown that maternal exposure to stress during pregnancy results in heightened cortisol release, hippocampal abnormalities, and an increase in fluctuating dermatoglyphic asymmetries and other dermatoglyphic abnormalities in offspring (see Walker and Diforio
[34] for a review). Research has also linked schizophrenia to prenatal exposure of the mother to stress
(52,
53). The present study showed a modest correlation between fluctuating dermatoglyphic asymmetries and cortisol slope. Presuming that the latter is an index of the HPA response to novelty, this may reflect the influence of prenatal neurodevelopment on both cortisol response and fluctuating dermatoglyphic asymmetries.
It is of interest that negative, but not positive, symptom ratings were correlated with cortisol level. Although this might be taken as further support for the notion that different neural processes underlie the two symptom dimensions, such a conclusion would be premature, because this study excluded subjects whose symptoms would meet criteria for an axis I disorder and, by definition, restricted the range of symptoms.
A remaining issue is whether minor physical anomalies, fluctuating dermatoglyphic asymmetries, and cortisol level are predictive of clinical course in individuals at risk for schizophrenia. As noted, recent studies indicate that dermatoglyphic abnormalities are linked with greater sensitivity to stress and that heightened cortisol values are associated with poorer 6-month outcome in clinic-referred children
(37). Follow-up of the present study group will allow us to determine whether there is an interactive effect of morphologic abnormalities and response to stress on clinical course.
The chief limitation of this study concerns the absence of outcome data. It is anticipated that some subjects in the schizotypal personality disorder group will continue to manifest the syndrome, some will improve, and some will develop axis I disorders, including schizophrenia. Follow-up of our study group will shed light on this issue. In addition, we cannot generalize the findings to adults with schizotypal personality disorder; it is possible that they are specific to early-onset schizotypal personality disorder.