Dopamine release in the ventral striatum, which includes the nucleus accumbens, follows drug and alcohol intake
(1,
2). It has been suggested that dopamine release in this central area of the brain reward system is strongly rewarding and that drug intake is reinforced by the hedonic affect elicited by drugs and drug-associated stimuli
(3). It has also been postulated that dopamine release in the nucleus accumbens attributes “incentive salience” to drug-associated stimuli, thus increasing the motivational value and attentional processing of drug cues, and it has been suggested that cue-induced dopamine release mediates drug “wanting” rather than drug “liking”
(4). This hypothesis was based on the observation of Schultz et al.
(5) that unexpected rewards and conditioned reward-indicating stimuli elicited dopamine signaling in the nucleus accumbens. When, however, primary rewards were fully expected because of the association with preceding reward-indicating cues, there was no increase in dopamine discharge. Schultz et al.
(5) suggested that dopamine discharge represents an error-detection signal that is increased upon confrontation with unexpected rewards or reward-indicating stimuli and decreased if conditioned cues are not followed by the reward.
Sustained cue-induced changes in dopamine discharge may code reward uncertainty and elicit a nonselective form of attention or arousal
(6). The ventral striatum is closely connected with the anterior cingulate
(7) and the dorsal striatum
(8). Cue-induced activation of the anterior cingulate and adjacent medial prefrontal cortex may mediate an attentional response to drug cues
(9,
10), while cue-induced dopamine release in the dorsal striatum can trigger relapse into drug-taking behavior
(11).
Alcohol, like other drugs of abuse, stimulates dopamine release and induces a down-regulation of striatal dopamine D
2 receptors
(12). Longitudinal studies have shown that D
2 receptor down-regulation is most prominent directly after detoxification and recovers during abstinence
(13,
14). Delayed recovery of central dopaminergic neurotransmission predicts a higher risk for relapse among detoxified alcoholics
(13–
15).
To further elucidate the association between individual differences in D
2 receptor dysfunction in the ventral striatum (particularly the nucleus accumbens), central processing of alcohol-associated cues, and alcohol craving, we used functional magnetic resonance imaging (fMRI) in combination with positron emission tomography (PET) to study detoxified male alcoholics and age-matched comparison subjects. We tested the hypothesis of Robinson and Berridge
(4) that low availability of D
2 receptors in the ventral striatum/nucleus accumbens mediates excessive attribution of incentive salience to alcohol-associated cues, causing a pathological “wanting” to consume alcohol. We also tested the hypothesis that in alcoholics a dysfunction of ventral striatum D
2 receptors interferes with a dopamine-dependent error detection signal
(5), so that alcohol-associated cues elicit excessive activation of the medial prefrontal cortex/anterior cingulate and dorsal striatum.
Results
The alcoholics had significantly lower D
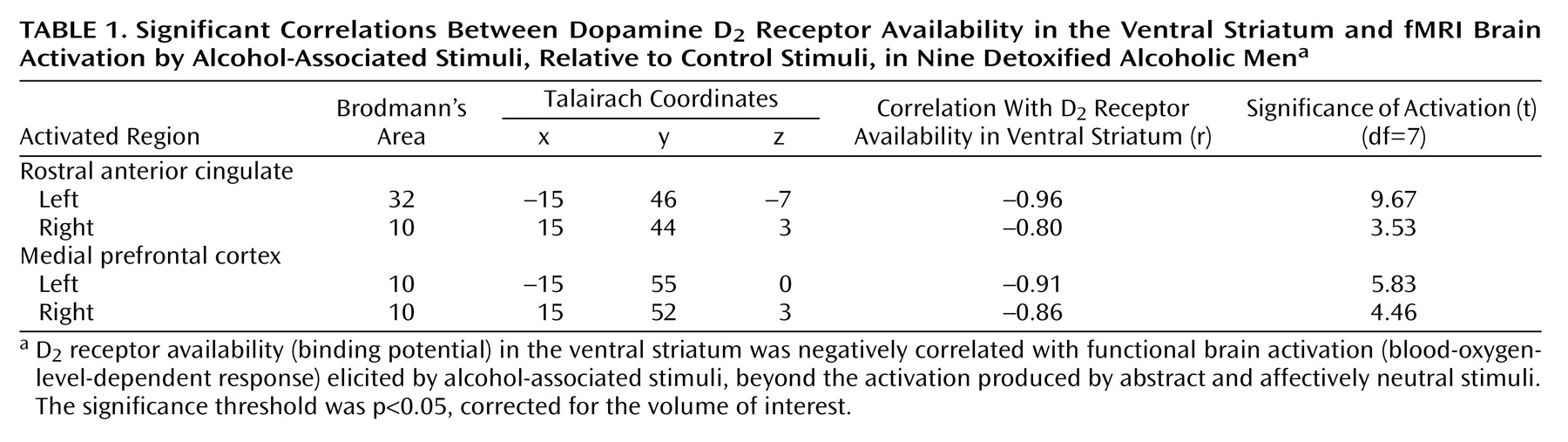
2 receptor availability than the healthy comparison subjects in the bilateral putamen and adjacent nucleus accumbens/ventral striatum (for Talairach coordinates –28, 10, –8, t=2.21; for coordinates 20, 12, –6, t=2.39; df=22, p<0.05, corrected for the striatal volume of interest). In the alcoholics, greater severity of alcohol craving was significantly and exclusively associated with low bilateral D
2 receptor availability in the nucleus accumbens/ventral striatum (
Figure 1).
The correlation was remarkably symmetrical for the nucleus accumbens/ventral striatum (for right-side Talairach coordinates 16, 14, –6, r=–0.91; for left-side coordinates –15, 14, –6, r=–0.90; N=11, p<0.05, corrected for striatal volume of interest; the finding remained statistically significant at p<0.05 when multiple comparisons in the whole brain were corrected). The radioligand [
18F]DMFP also binds to D
2 receptors outside of the striatum to a low but significant extent
(19). However, alcohol craving was not associated with D
2 receptor availability outside of the ventral striatum. Among the healthy comparison subjects, no significant correlation was observed between alcohol craving and D
2-like receptor availability.
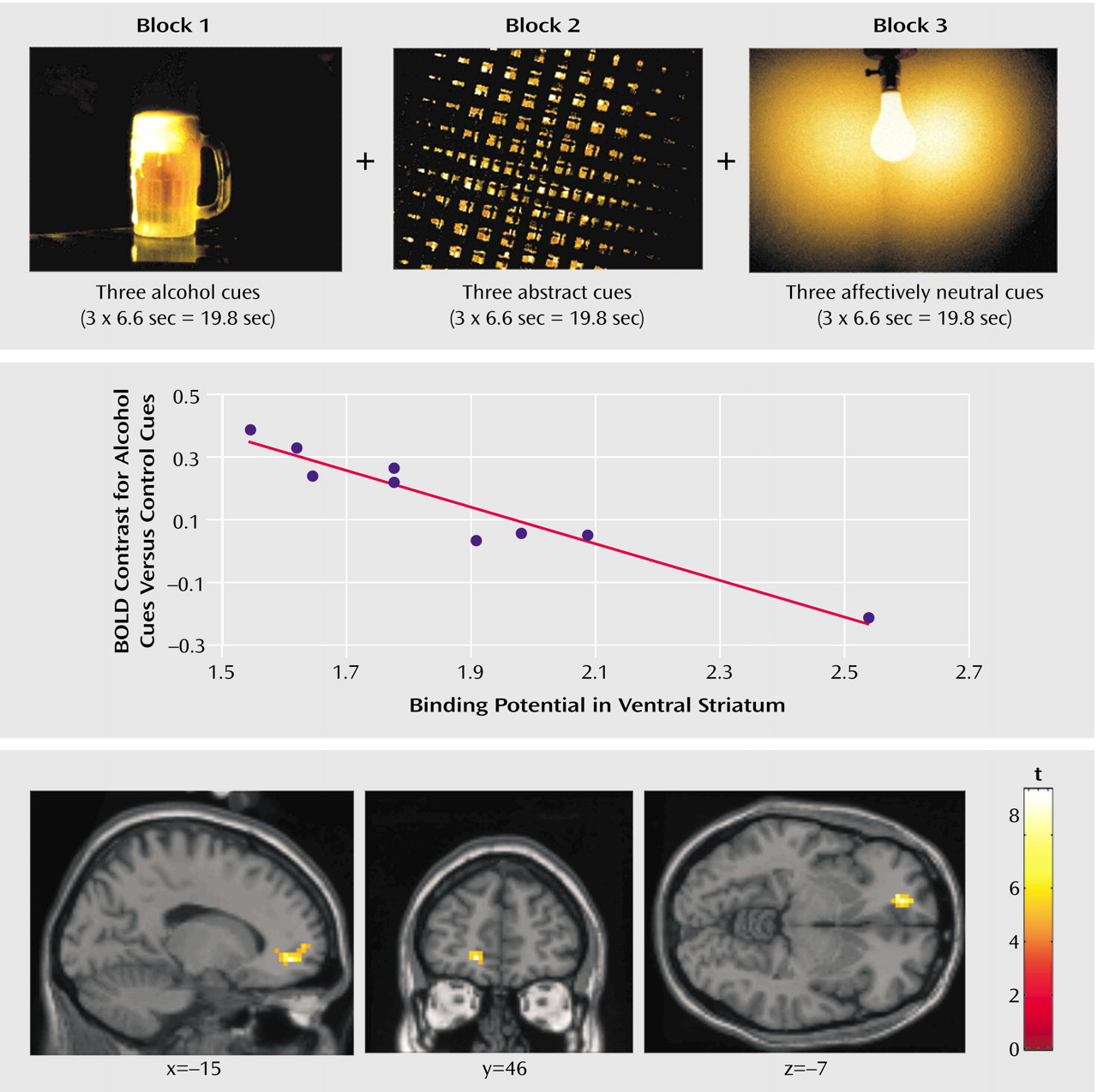
The fMRI images showed that activation by visual alcohol-associated stimuli, beyond that produced by the control stimuli, was significantly greater for the alcoholics than for the healthy volunteers in the left medial prefrontal cortex (Brodmann’s area 10, coordinates –18, 52, –8, t=2.05) and left caudate (coordinates –6, 9, 8, t=2.54) (df=19, all p<0.05 corrected for volume of interest). In the alcoholics, D2 receptor availability in the ventral striatum was significantly and negatively correlated with cue-induced fMRI activation in the left medial prefrontal cortex (Brodmann’s area 10, coordinates –18, 52, –8: r=–0.82, N=9, p<0.05 after Bonferroni correction) but not in the left caudate (coordinates –6, 9, 8: r=0.16, N=9, p=0.66). No significant associations were observed in the comparison subjects in these regions of interest.
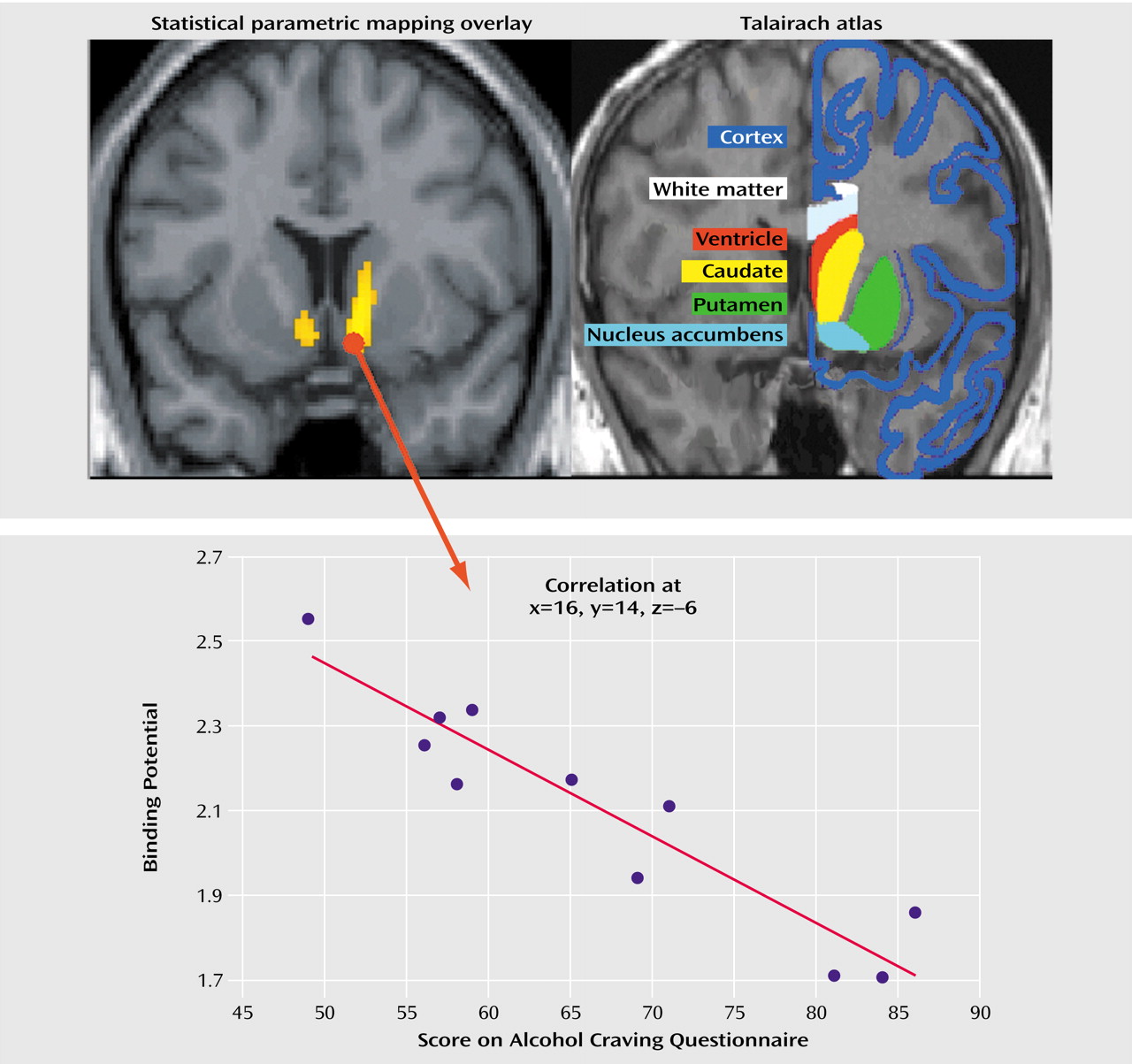
Using SPM 99, we also assessed the association between the availability of D
2 receptors in the nucleus accumbens/ventral striatum and brain activation elicited by alcohol versus control cues. We observed that among the alcoholics, low striatal D
2 receptor availability was associated with greater cue-induced activation of the bilateral rostral anterior cingulate (Brodmann’s area 32/10) and medial prefrontal cortex (Brodmann’s area 10) (
Table 1 and
Figure 2).
Among the healthy comparison subjects, D2 receptor availability was not associated with cue-induced brain activation in the cingulum or medial prefrontal cortex.
We did not observe significant correlations between the main outcome variables of the study (severity of alcohol craving, mean D2 receptor availability in the ventral striatum, cue-induced activation of the medial prefrontal cortex) and potentially confounding variables: smoking, age at onset of alcohol dependence, severity of alcoholism, lifetime alcohol intake, number of previous detoxifications, or range of time between last drink and scanning (r=0.46–0.01, p>0.10 in all cases, N=11 for alcohol craving and mean D2 receptor availabilitiy, N=9 for cue-induced brain activation).
Discussion
This study revealed two major findings. First, it lends direct support to the hypothesis of Robinson and Berridge
(4) that dopamine dysfunction in the ventral striatum, which includes the nucleus accumbens, is associated with alcohol craving. In previous studies, individual differences in the speed of recovery of central dopaminergic neurotransmission predicted the relapse risk of alcoholics during early abstinence
(13–
15). In accordance with these observations, we found considerable variation in D
2 receptor availability in the ventral striatum/nucleus accumbens of alcoholics who had abstained from alcohol for 3 weeks. It is currently not known whether the low D
2 receptor availability observed represents a counteradaptive down-regulation triggered by chronic alcohol-associated dopamine release or whether it is due to alcoholism-related neurotoxicity. In any case, our study suggests a potential mechanism by which persistently low levels of D
2 receptors in the ventral striatum may contribute to excessive alcohol urges and the motivation for relapse. We presented alcohol-associated pictures in a scanner while the alcohol-dependent patients were participating in a detoxification program. These patients were well informed that no alcoholic beverages were available during or after the brain imaging study. Therefore, the pictures of alcoholic beverages did not indicate the availability of an alcohol or drug reward. In accordance with this setting, the healthy comparison subjects showed no activation of the anterior cingulate or frontal cortex. In contrast, alcohol-associated stimuli elicited increased activation of the medial prefrontal cortex and caudate among the alcohol-dependent patients. The strength of the activation of the medial prefrontal cortex (Brodmann’s area 10) and the adjacent anterior cingulate was inversely correlated with the availability of dopamine receptors in the ventral striatum. A lack of D
2 receptors in the ventral striatum, particularly the nucleus accumbens, may interfere with the normal role of dopaminergic neurotransmission as an error-detection signal that indicates the availability of reward
(5). Patients may thus lack a dopamine-dependent striatal gating function
(39) that filters out stimuli that are not followed by reward
(40). As a consequence, alcohol-associated stimuli that were not followed by alcohol intake in this study elicited excessive activation of the medial prefrontal cortex and anterior cingulate. The anterior cingulate is a part of the medial prefrontal cortex, which is thought to be important in attention
(41, pp. 175–176), conditioned drug seeking
(10,
42), and craving
(29,
30,
33). Cue-induced stimulation of the medial prefrontal cortex and anterior cingulate may thus reflect enhanced attentional processing of alcohol-related stimuli
(41, p. 175), which become ”wanted” and elicit drug craving
(4).
Some potential limitations of the study should be addressed. The patients rested in the PET scanner with their eyes closed. While they were told to relax, some of them may have experienced various degrees of alcohol craving, which might affect acute dopamine release in the ventral striatum and interact with radioligand binding to striatal D2 receptors. Correlations do not imply causation, and the exact nature of the interaction between D2 receptor availability in the ventral striatum and alcohol craving remains to be elucidated in further studies.
This study showed that alcohol craving is associated with a low availability of D
2 receptors in the bilateral ventral striatum, including the nucleus accumbens, a central area of the brain reward system. Among alcoholics but not healthy comparison subjects, D
2 receptor availability in the bilateral ventral striatum was negatively correlated with cue-induced activation of the frontal cortex and cingulum. Reinstatement of alcohol abuse may depend on dopaminergic dysfunction
(12,
14) in the ventral striatum that promotes cue-induced activation of a neuronal network associated with attention, drug reward, and craving
(9,
28,
29,
33). These observations indicate that neuroreceptor PET and fMRI can successfully be combined to reveal the in vivo interaction between neurotransmission and the neural networks associated with emotion and attention. They point to a neurobiological correlate of alcohol urges and support the hypothesis that dopaminergic dysfunction in the brain reward system plays a key role in alcohol craving among humans.