In the general population, minor depressive disorder is estimated to have a 1-month point prevalence of approximately 2% (Epidemiologic Catchment Area study)
(6) and 10% lifetime prevalence (National Comorbidity Survey)
(5). In a large clinical study of patients with major depressive disorder, minor depressive symptoms were observed almost twice as frequently as major depressive symptoms during the long-term (average 12-year) course of illness
(8). These reports indicate that minor depressive disorder is not an evanescent, nonspecific variation of normal mood but, rather, has the characteristics of a clinically significant medical condition
(9). Understanding the course and response to treatment of minor depressive disorder has become even more important as opinion in psychiatry has evolved to accept that depressive subtypes included in the official diagnostic nomenclature (DSM-IV, ICD-10) may alternatively be conceptualized as part of a dimensional continuum of depressive symptom severity in patients with major depressive disorder
(5,
6,
8,
10–12). Thus, depressive symptoms at the major, minor, dysthymic, or subsyndromal levels clearly fluctuate within the individual major depressive disorder patient, as phases of illness activity and intensity vary over their lifetime
(13).
Regrettably, despite the high prevalence of minor depressive disorder
(4–
6), the associated psychosocial impairment
(4–
7), and the growing recognition of its public health importance, there are few published treatment studies of minor depressive disorder. Two psychotherapy studies had positive results
(14,
15), but three antidepressant medication studies, which varied in experimental rigor or used an older antidepressant, had mixed results
(16–
18). More recently, Williams and colleagues
(19) compared the effects of a selective serotonin reuptake inhibitor (SSRI), problem solving therapy, and placebo in older individuals with minor depressive disorder identified through primary care facilities. They reported that the patients treated with the SSRI experienced more rapid improvement of depressive symptoms than those who received either placebo or the problem solving therapy. There remains a need for rigorous studies assessing the response of patients with minor depressive disorder to all forms of therapeutic intervention.
This article presents the results of a randomized, placebo-controlled, double-blind study conducted to test the effects of 12 weeks of fluoxetine treatment on depressive symptoms and psychosocial function in a large group of outpatients with carefully diagnosed minor depressive disorder. This investigation focused on the treatment of acute minor depression (symptoms of at least 14 days’ duration), not chronic minor depression (symptoms of at least 2 years’ duration), which is more accurately diagnosed as dysthymia. Our primary hypothesis was that fluoxetine-treated subjects with minor depressive disorder would have significantly greater improvement on the 30-item Inventory of Depressive Symptomatology, clinician-rated version
(20), than subjects with minor depressive disorder who received placebo. Our secondary hypotheses were that fluoxetine-treated subjects with minor depressive disorder, compared with subjects who received placebo, would have significantly greater improvement on other clinical measures such as the 17-item, 21-item, and 28-item Hamilton Depression Rating Scale
(21), the Clinical Global Impression (CGI) severity scale
(22), and the Beck Depression Inventory
(23), as well as greater improvement on psychosocial measures such as Global Assessment of Functioning Scale (GAF) (DSM-IV, p. 32) and scales within the Medical Outcomes Study 36-Item Short-Form Health Survey (MOS-36)
(24).
Method
Subject Recruitment and Evaluation
Subjects were recruited from three sites (University of California, San Diego; University of Pittsburgh; and University of Texas, Southwestern) after raters underwent an intensive 3-day training session in which they attained good interrater reliability on the diagnostic criteria and rating instruments used in the investigation. After a telephone screening, subjects who gave written informed consent underwent a physical examination and diagnostic evaluation with the Structured Clinical Interview for DSM-IV (SCID)
(25). The initial evaluation also included the depression module of the National Institute of Mental Health Diagnostic Interview Schedule (DIS)
(26); the GAF; the MOS-36; the 28-item Hamilton Depression Rating Scale, which was also scored for the 17-item and 21-item versions; the 14-item Hamilton Anxiety Rating Scale
(27); the 30-item Inventory of Depressive Symptomatology, clinician-rated version; the 21-item Beck Depression Inventory; the 90-item Hopkins Symptom Checklist (SCL-90)
(28); the CGI severity scale; a demographic assessment; and Research Diagnostic Criteria for family history diagnoses
(29).
Subjects were reevaluated weekly during a 4-week placebo lead-in period and while in the 12-week treatment phase with the DIS, Inventory of Depressive Symptomatology, 28-item Hamilton Depression Rating Scale, MOS-36, GAF, CGI severity scale, and CGI improvement scale. Clinician- and patient-rated CGI improvement ratings were also made at each weekly visit. The 21-item Beck Depression Inventory was administered at the initial and final study visit of the 12-week treatment period.
Study Criteria
As presented more fully in our prior paper
(9), we adopted the DIS as our primary diagnostic tool for defining minor depressive disorder and required a level of functional disability based on the GAF and at least one of two MOS-36 subscales (
Appendix 1 shows the criteria for minor depressive disorder). To qualify as having “confirmed minor depressive disorder” for the treatment phase of the study, patients needed to meet these criteria at their initial diagnostic visit and at least 3 of the 4 subsequent weeks, including the last 2 weeks of the placebo lead-in period. If, at any time, a subject had developed five or more symptoms of a major depressive episode, as assessed with the DIS depression section, the depression module of the SCID was administered to determine whether the patient had progressed to major depressive disorder—in which case the patient was released from this study and treated outside the protocol.
Subjects were eligible for the study if they were at least age 18 years, healthy, conversant in English, and willing to participate in the full 40-week study consisting of a 4-week single-blind placebo lead-in period, the 12-week double-blind acute treatment period reported on here, and a 24-week crossover treatment period. Subjects also had to have normal results on a physical examination and on laboratory tests, including CBC, urine toxicology screen, urine analysis, and serum chemistries for hepatic and renal function.
Patients were excluded from the study if they had major depressive disorder or dysthymic disorder currently or within the past 2 years; major depressive disorder in partial remission; a loss of a loved one or significant other within the past year that could account for the mood disorder; serious suicidal risk; substance or alcohol abuse or dependence within the past year; a current diagnosis of any axis I disorder; a lifetime diagnosis of bipolar disorder (type I), borderline personality disorder, antisocial personality disorder, psychotic disorder, organic mood disorder, organic psychotic disorder, or schizophrenia; use of psychotropic drugs except chloral hydrate within 7 days of study entry or use of a monoamine oxidase inhibitor within 14 days of starting active treatment; the presence of a serious medical condition not presently stabilized; seizure disorder within the past year; a history of severe allergies or multiple adverse drug reactions; previous nonresponse or adverse reaction to fluoxetine; or previous participation in a fluoxetine study. Patients who met all of the inclusion criteria were randomly assigned to receive fluoxetine or placebo treatment for this 12-week, double-blind investigation.
Dosing Schedule
Patients were instructed to take the study medication each morning, beginning with one 10-mg capsule of fluoxetine or a look-alike placebo. After 2 weeks, patients without intolerable side effects and without significant improvement were instructed to increase the dose to two capsules (20 mg/day of fluoxetine or placebo). For patients who experienced adverse effects, the dose could be titrated down once to 10 mg/day and then back up if symptoms persisted. Chloral hydrate was the only other psychotropic drug allowed during the treatment period. Nonpharmacologic antidepressant treatments, such as light therapy and psychotherapy, were also prohibited.
Statistical Analysis
Demographic characteristics, baseline clinical characteristics, time in study, and adverse events of the fluoxetine and placebo groups were compared by using analysis of variance (ANOVA) for continuous measures and chi-square tests for categorical variables. Ordinal variables (e.g., CGI scale scores) and extremely nonnormally distributed variables (e.g., MOS-36 scores) were analyzed with the Wilcoxon rank sum test.
A much longer than usual placebo lead-in period was used in this study to identify placebo responders and to highlight true drug response. For patients who participated in the acute treatment phase, depression and psychosocial functioning scores from the start and end of the placebo lead-in period were analyzed by repeated-measures ANOVA.
The main hypothesis, that fluoxetine would be superior to placebo in ameliorating minor depressive symptoms, was tested with a last-observation-carried-forward repeated-measures ANOVA that used the SAS GLM procedure
(30) to analyze changes in Inventory of Depressive Symptomatology scores from baseline to the last visit in the acute treatment phase. Similar analyses were conducted to test secondary hypotheses concerning differences between treatment groups on all other measures of depressive symptom severity and functioning. Complementary and confirmatory information about the treatment effects of fluoxetine versus placebo was obtained by comparing
rates (slopes) of improvement from mixed (random-effects) regression analysis conducted on all weekly measures of depressive symptoms severity and functioning over the 12-week treatment period. Mixed regression analysis of continuous measures was performed with MIXREG
(31) software, and analysis of ordinal outcomes (MOS-36 and CGI severity scale scores) was performed with the MIXOR
(32) program. In the mixed regression analyses, each dependent variable was modeled on the basis of a random intercept term, a random effect representing time (weeks) in treatment, and fixed effects representing treatment (fluoxetine versus placebo), as well as a treatment-by-week interaction term for group differences in outcome over the course of the study. A first-order autoregressive process (a stationary AR
[1] process) was used to characterize the autocorrelation structure. Initial analyses were conducted to test for treatment-by-time-by-site and treatment-by-time-by-gender interactions. Since these interactions were nonsignificant, site and gender were dropped from the ANOVA and mixed regression models that are reported here. A two-tailed alpha level of p=0.05 was used to determine statistical significance. It was not appropriate to make an adjustment for the multiple dependent variables used in this study because of the high correlations among the different measures of depression or psychosocial functioning.
Results
Patient Characteristics
Of the 226 patients who qualified for the study, 64 were not randomly assigned to treatment groups after the 4-week placebo lead-in period for the following reasons: onset of a major depressive episode (N=5), failure to meet minor depressive disorder criteria (N=14), experience of adverse effects (N=4), inability to be contacted (N=6), patient’s decision to withdraw (N=22), contraindicated medical condition (N=10), or other protocol violations (N=3). Of the 162 patients who were randomly assigned to treatment groups (N=81 for the fluoxetine group; N=81 for the placebo group), 59.3% were female, and 90.1% were Caucasian; the average age was 43.5 years (SD=11.7, range=18–72).
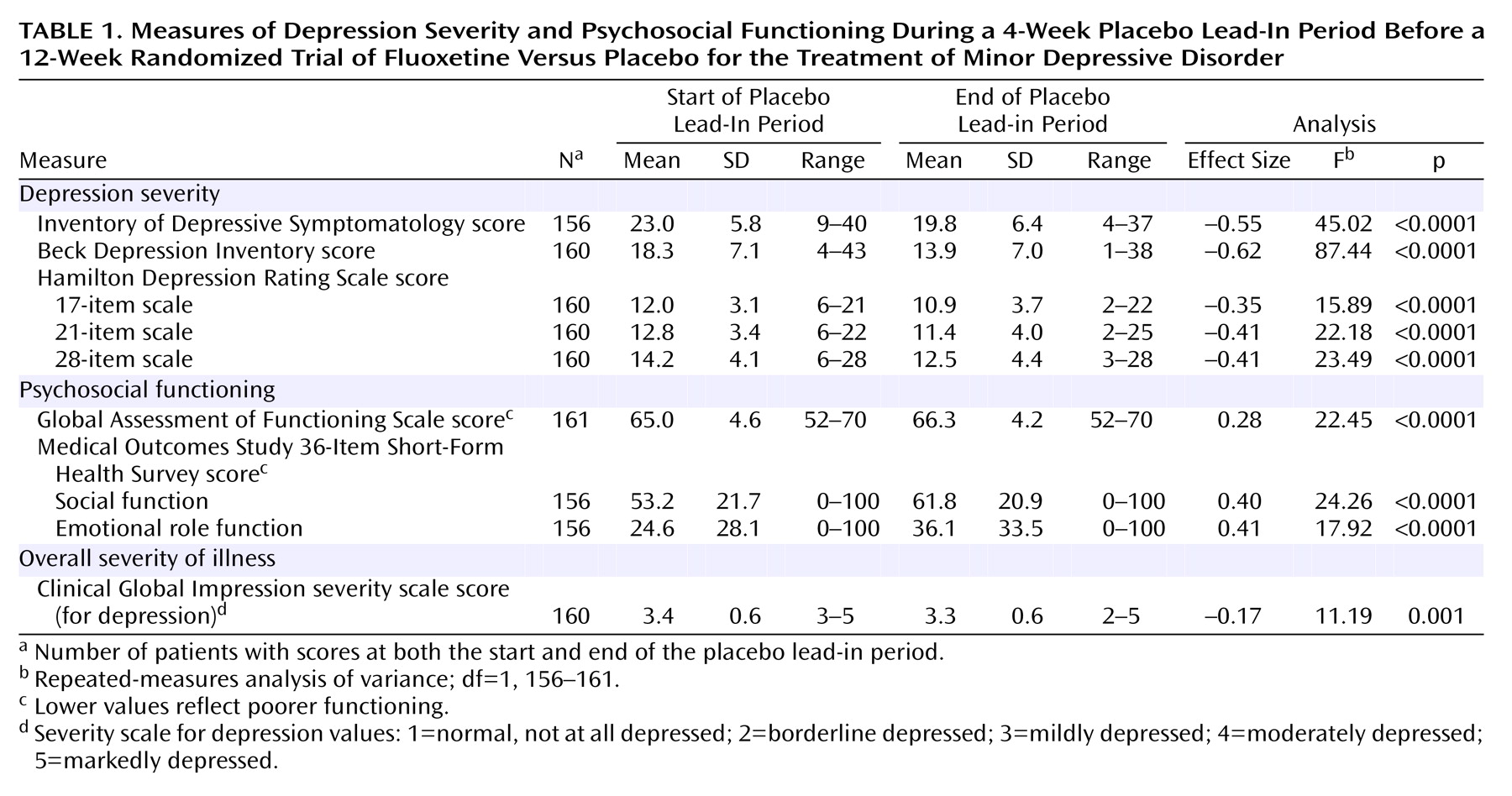
Depression Severity and Psychosocial Function During the 4-Week Placebo Lead-In
As shown in
Table 1, the subjects with minor depressive disorder were mildly to moderately depressed at intake and had a mild to moderate level of psychosocial impairment. During the single-blind placebo lead-in period, patients improved significantly (p<0.0001, repeated-measures ANOVA) on all measures of depression severity (range of effect sizes=–0.35 to –0.62) as well as on all measures of psychosocial functioning (range of effect sizes=0.28 to 0.41) (
Table 1). At the start of double-blind treatment, the fluoxetine and placebo groups were not significantly different on any demographic characteristics, clinical measures, or psychosocial measures.
Study Completion Status
Seventy-three percent (59 of 81) of each group completed 12 weeks of treatment. The mean time in treatment was 9.8 weeks for both groups (median for both groups=12 weeks). The 22 dropouts in each treatment group were due to: lack of efficacy (fluoxetine group: N=6; placebo group: N=9), adverse events (fluoxetine: N=3; placebo: N=4), loss to contact (N=3 in each group), moving away (fluoxetine: N=2; placebo: N=1), scheduling problems (fluoxetine: N=7; placebo: N=3), development of a major depressive episode (fluoxetine: N=0; placebo: N=1), and protocol violations (N=1 in each group).
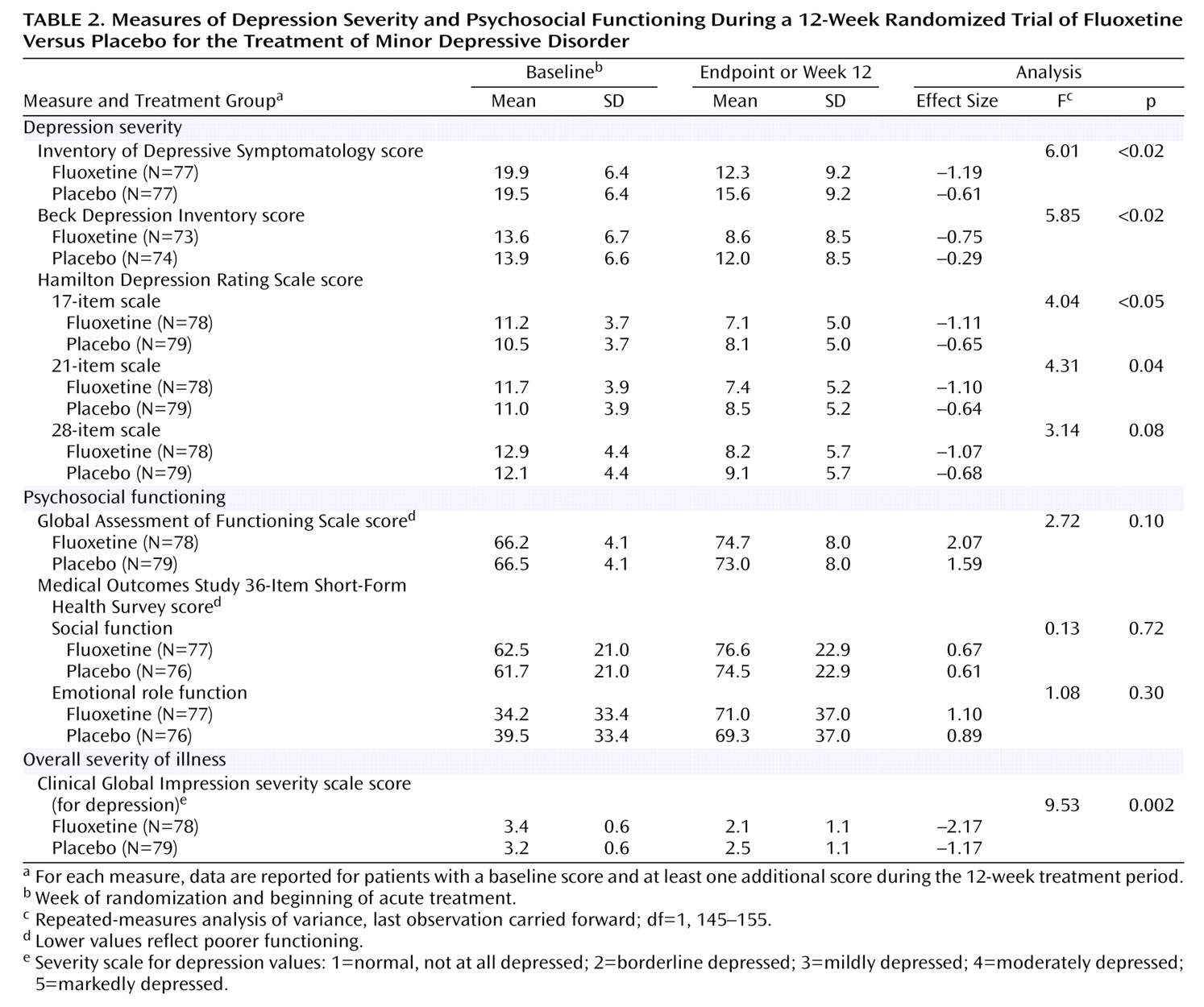
Depression Severity During the 12-Week Treatment Trial
Repeated-measures ANOVA showed that from baseline until the last treatment visit, the fluoxetine group improved significantly more than the placebo group on four of five measures of depressive symptom severity (
Table 2). For the clinician-rated Inventory of Depressive Symptomatology score, which was defined a priori as the primary outcome measure for this study, the treatment effect size for fluoxetine was approximately double that for placebo (–1.19 versus –0.61). Compared with the placebo group, the subjects treated with fluoxetine also had significantly greater improvement as measured by the self-rated Beck Depression Inventory and the 17-item and 21-item versions of the Hamilton Depression Rating Scale but not the 28-item version. Secondary analysis with mixed (random-effects) regression analysis showed a statistically greater
rate of improvement in the fluoxetine group than in the placebo group in Inventory of Depressive Symptomatology scores (z=2.40, p<0.02), 17-item Hamilton Depression Rating Scale scores (z=2.06, p=0.04), and 21-item Hamilton Depression Rating Scale scores (z=2.19, p<0.03). The fluoxetine group’s greater rate of improvement in 28-item Hamilton Depression Rating Scale scores approached significance (z=1.93, p=0.053). Change in Beck Depression Inventory scores was not analyzed by mixed regression analysis because this instrument was administered only at baseline and at the last visit, rather than on a weekly basis.
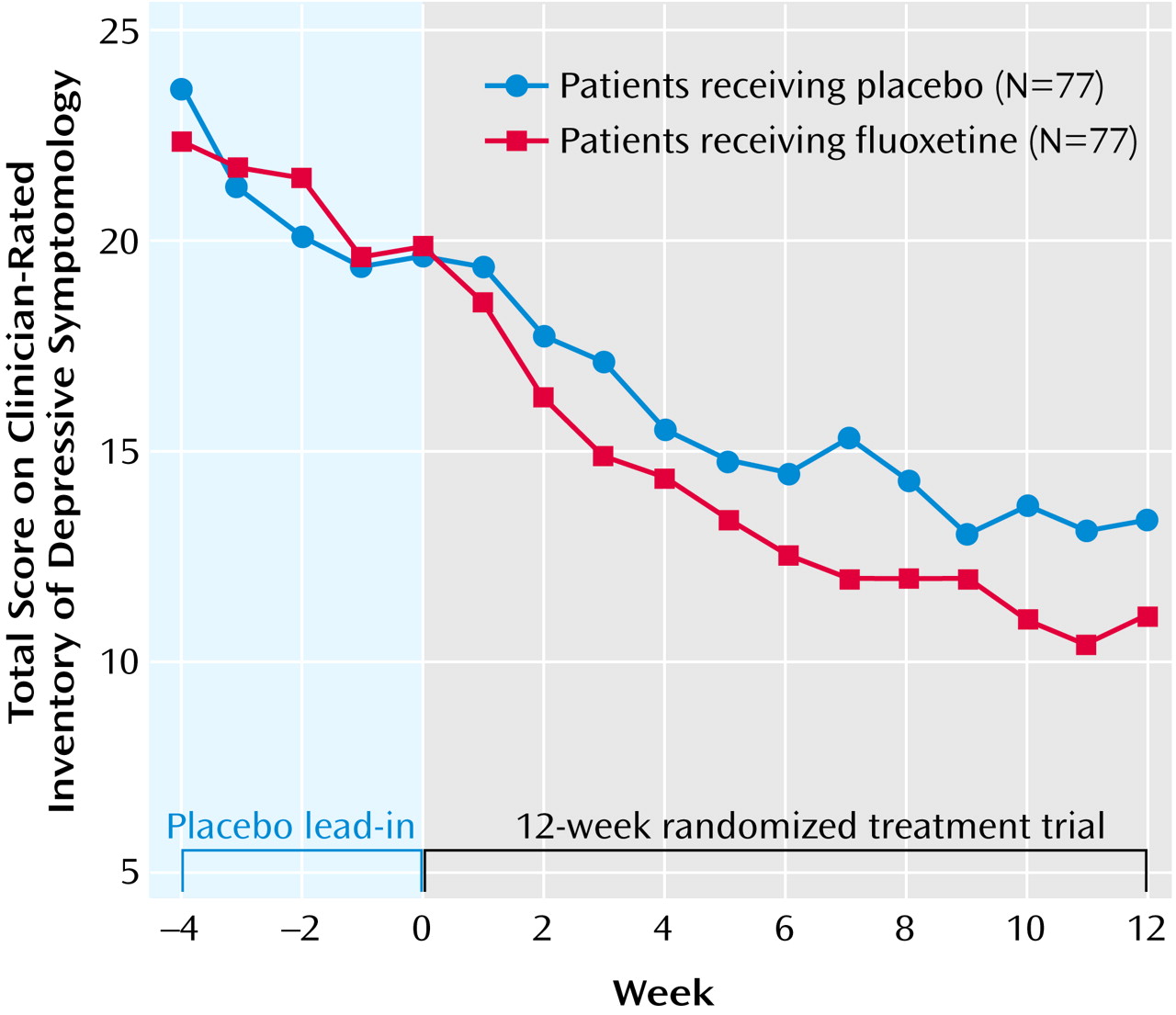
Figure 1 is a plot of the mean weekly Inventory of Depressive Symptomatology scores for each treatment group. Both groups showed improvement during the placebo lead-in period. Week-by-week repeated-measures ANOVAs showed that the difference in improvement between the fluoxetine and placebo groups became statistically significant at 7 weeks of treatment. Patterns of response similar to that seen in
Figure 1 were found for other measures of depressive symptom severity.
Psychosocial Functioning During the 12-Week Treatment Trial
Repeated-measures ANOVA showed that the fluoxetine group did not improve more than the placebo group on any of three measures of psychosocial functioning (
Table 2). The secondary analysis with mixed regression, however, showed that the
rate of improvement in the GAF score in the fluoxetine group was significantly greater than that in the placebo group (z=2.10, p<0.04).
Clinical Global Impression Measures During the 12-Week Treatment Trial
At baseline, the fluoxetine and placebo groups had similar CGI severity ratings, ranging from “mildly ill” to “markedly ill.” At exit, 40.5% of the fluoxetine patients, compared to 24.1% of the placebo group, were rated as “normal, not at all depressed” (χ
2=6.63, df=1, p=0.01). Repeated-measures ANOVA showed a significantly greater improvement on the CGI severity rating for the fluoxetine group than for the placebo group (
Table 2). Ordinal mixed regression analysis showed a statistically greater
rate of improvement for the fluoxetine group than for the placebo group on this measure (z=2.56, p=0.01). The results of a Wilcoxon rank sum test showed that clinicians’ CGI improvement ratings at the last assessment were significantly better for the fluoxetine group than for the placebo group (p<0.04); the CGI improvement ratings made by the patients were better in the fluoxetine group than in the placebo group, but the difference only approached significance (p=0.057).
Other Measures During the 12-Week Treatment Trial
Repeated-measures ANOVA showed that the fluoxetine and placebo groups did not differ significantly in improvement as measured by the SCL-90 total score (F=2.30, df=1, 151, p=0.13) or the Hamilton Anxiety Rating Scale score (F=0.56, df=1, 155, p=0.46).
Adverse Events
After receiving 10 mg/day of fluoxetine or placebo for 2 weeks, 74 patients (91.4%) in each group were treated with 20 mg/day. By their last visit, each group spent about 70% of their study weeks at this target dose. Adverse events were recorded on the basis of spontaneous reports from study participants. During the placebo lead-in period, 92.6% of the patients in each group reported one or more adverse events. The mean number of adverse events during the 12-week course of treatment was 5.2 (SD=3.6) in the fluoxetine group and 4.6 (SD=3.6) in the placebo group (z=1.45, p=0.15, by Wilcoxon rank sum test). There were no significant differences between the fluoxetine and placebo patients on the worst reported level of severity of adverse events (z=0.46, p=0.65). The only significant difference between the treatment groups was that insomnia was experienced by a greater percentage of the fluoxetine patients than of the placebo patients (24.7% versus 12.4%; χ2=4.09, df=1, p<0.05). No differences were found in sexual side effects, although these effects tend to be underestimated by the spontaneous report method. In summary, apart from the between-group difference in the rate of insomnia, the two groups were similar in the number and severity of adverse events as spontaneously reported during treatment.
Discussion
This report describes a carefully controlled investigation of the effects of 12 weeks of fluoxetine, compared with placebo, in a large number of patients with minor depressive disorder (N=162) selected to be clinically homogeneous. The mean baseline depression rating scale scores indicated that the patients had mild to moderate depressive symptom severity. The mean baseline psychosocial function scores were in the moderately impaired range.
This study revealed fluoxetine to be significantly superior to placebo in reducing depressive symptom severity but not in reducing general measures of psychopathology (e.g., SCL-90 score) or anxiety (Hamilton Anxiety Rating Scale score). Although the changes in depression severity scores were small, they were consistently and significantly greater for the fluoxetine group than for the placebo group. The differences in effect sizes favored fluoxetine by a large margin. Improvement in global psychosocial functioning (GAF score) was not significantly better for the fluoxetine group, compared to the placebo group, in repeated-measures last-observation-carried-forward ANOVA; it was, however, significantly better in the fluoxetine group in mixed regression analyses that reflected the
rate of change during the entire treatment period rather than change based only on the difference between baseline and final scores. The MOS-36 social role function and emotional role function scores did not distinguish the fluoxetine group from the placebo group by either method of analysis. These specific areas of psychosocial function may not have been affected by fluoxetine, or there may have been a delay in improvement similar to that reported previously for major depressive episodes
(33). It has also been reported that specific domains of psychosocial function are affected differently by various levels of depressive symptom severity
(7). If so, a 12-week treatment period may be too brief to detect improvement in specific areas of psychosocial function. Alternatively, there may not have been sufficient baseline impairment in psychosocial function for a significant between-group difference to develop during the treatment period.
The fluoxetine and placebo groups had identical durations of time in treatment and identical study completion rates. Data on adverse events were obtained by spontaneous patient report, and, except for insomnia, no adverse event, including sexual side effects, had significantly different rates of occurrence in the two treatment groups.
We anticipated that the placebo response rate for patients with minor depressive disorder might be higher than that reported for patients with major depressive episodes. To control for placebo response, strict a priori diagnostic criteria were used. In addition, an unusually long 4-week single-blind placebo lead-in was used to identify placebo responders before randomization. It is interesting to note that only 19 (8.4%) of the initial 226 minor depressive disorder patients failed to meet the diagnostic criteria for minor depressive disorder after 4 weeks of taking placebo (14 of the 19 patients improved to the point of no longer meeting the criteria for minor depressive disorder, and five worsened to the point of meeting the criteria for a major depressive episode). This finding provides impressive support for the stability of the case definition and the nontransient nature of minor depressive disorder illness.
Because the standard clinical depression rating scales (e.g., Hamilton Depression Rating Scale and Beck Depression Inventory) were developed and oriented to monitor onset and resolution of major depressive episodes, we were concerned that these instruments might not be sensitive to treatment-related changes in minor depressive disorder. Further, we reported previously that minor depressive disorder patients experience more cognitive depressive symptoms, rather than neurovegetative or reverse neurovegetative signs and symptoms, which are overrepresented in the standard instruments
(9). Because the Inventory of Depressive Symptomatology has the capacity to accurately measure depressive symptoms ranging from minimal to severe
(20,
34) and has response options that reflect both the intensity and the frequency of symptoms, it was chosen as the primary outcome measure for this study. Other standard instruments developed for the assessment of symptoms of major depression—namely the 28-item Hamilton Depression Rating Scale (scored for 17-item and 21-item versions), the Beck Depression Inventory, and the CGI severity scale—were included as secondary or confirming outcome measures but were not expected to be as sensitive to treatment change as the Inventory of Depressive Symptomatology. Although the effects of fluoxetine were consistently superior to that of placebo, as measured by all but one of the secondary outcome measures of depressive symptom severity, the Inventory of Depressive Symptomatology proved to be the most sensitive clinician-rated instrument for assessing therapeutic response in minor depressive disorder.
Findings from this rigorously controlled study indicate that minor depressive disorder can be treated successfully with fluoxetine, but full normalization of associated psychosocial function may require longer treatment. It is also likely that other SSRIs or newer non-SSRI antidepressants might produce similar results under similar experimental conditions; these hypotheses await further study.
These data could have been influenced by several factors. First, the fluoxetine doses were in the lower range (i.e., 10–20 mg/day). It is possible that higher therapeutic doses (i.e., ≥40 mg/day) could have produced more optimal minor depressive disorder response. Second, the available standardized depression rating scales used, even the Inventory of Depressive Symptomatology, were all validated for major, not minor or subthreshold depression. Further analysis of data from this study will focus on how these scales perform as measures of minor depressive disorder severity, stability, and remission status. Third, by the end of the 4-week placebo lead-in period, a few patients had low scores on the depression rating scales (e.g., Inventory of Depressive Symptomatology, 17-item Hamilton Depression Rating Scale) but still met the criteria for minor depressive disorder defined by thresholds on the DIS, GAF, and MOS-36 (
Appendix 1). Fourth, patients were seen at weekly intervals, which probably contributed to the high nonspecific positive response rate in the placebo group.
Prior multicenter studies of treatment efficacy in major depression have shown site differences, which we did not find in this study of minor depression. This difference may have been the result of the intensive prestudy efforts to train raters in the standardized diagnosis, treatment, and assessment procedures, which were then applied by a highly experienced group of collaborating investigators.
A previous report by Paykel et al.
(17) concluded that minor depression (Hamilton Depression Rating Scale values ranging from 6 to 12) does not respond significantly better to the antidepressant amitriptyline (administered in a median dose of 125 mg/day) than to placebo. The implication is that the severe form of depression (major depression) responds significantly to pharmacological intervention, compared to nonspecific (i.e., placebo) measures, while the milder form (minor depression) does not. In the absence of other definitive studies, this one investigation has played a role in influencing thinking and therapeutic practice since the late 1980s
(35). The current study indicates that this assumption should be revised, since pharmacologic treatment with fluoxetine had clear benefit over nonspecific intervention for minor depressive disorder.
This study demonstrates that minor depressive disorder symptoms decrease and patients’ suffering is reduced with 12 weeks of SSRI treatment. Additional research investigating other antidepressants and psychotherapies in large well-characterized groups of subjects would be useful.