The antisaccade task has been studied as a measure of frontostriatal integrity. In this task, a reflexive saccade to a peripheral stimulus must be inhibited. Instead, a voluntary saccade is performed in the opposite direction. In performance of the antisaccade task, schizophrenia patients display increased error rates
(1). Functional neuroimaging studies of antisaccade performance have primarily implicated frontal and supplementary eye fields, the dorsolateral prefrontal cortex, thalamus, and striatum in healthy populations
(1) and have reported decreased activity in the dorsolateral prefrontal cortex, anterior cingulate, insula, and striatum of schizophrenia patients relative to healthy individuals or well-performing schizophrenia patients
(2–
4).
Some neurocognitive measures have shown a relationship with regional gray matter volumes in schizophrenia patients and healthy subjects
(5), although altered structure-function relationships in first-episode psychosis have also been observed
(6). This study is the first to our knowledge to explore the volumetric neural correlates of antisaccade performance.
Method
Twenty first-episode psychosis patients (mean illness duration=7.06 weeks, SD=6.75) and 18 healthy comparison subjects took part. At the time of study entry, seven patients were receiving typical antipsychotics, and four were receiving atypical antipsychotics; the remaining nine had not been treated with antipsychotic medication. Symptoms were assessed by three raters (intra- and interrater reliability >0.90) using the Positive and Negative Syndrome Scale (scored 1–7), which yielded scores for negative symptoms (mean=20.47, SD=5.83), positive symptoms (mean=21.42, SD=4.93), and general psychopathology (mean=42.89, SD=7.57) as well as a total score (mean=84.79, SD=13.87). Exclusion criteria for all subjects were neurological disorder and substance abuse. Comparison subjects did not have a current or past DSM-IV axis I or axis II diagnosis or history of psychosis among first-degree relatives. Subjects provided written informed consent. The local ethics committee approved the study procedures.
Light-emitting diodes were presented 200 cm from participants (target angle=0.15º) in a darkened room. There were four practice and 16 experimental trials (central stimulus=1000 msec, peripheral stimulus=1000 msec at ±15°). Instructions were to look at the target when in the center and to the mirror image location when it stepped to the periphery. Head movements were minimized using a chin rest. Left eye movements were recorded by using infrared oculography (sampling frequency=500 Hz) (Skalar Medical BV, Delft, the Netherlands). Saccades (velocity ≥30°/second; amplitude ≥1.5°; latency ≥100 msec, to exclude predictive saccades) were classified by two raters (inter- and intrarater reliability >0.90) as antisaccades (eye movement away from target), errors (eye movement toward target), or corrections (eye movement away from target following an error). The error rate (number of errors over number of valid trials), the amplitude gain percentage (antisaccade amplitude over target amplitude), latency (msec), and correction rate were measured.
Three-dimensional T
1-weighted spoiled gradient recall acquisition scans were acquired on a 1.5-T GE Signa Advantage in the axial plane (TE=2.2 msec, TI=300 msec, TR=11.3 msec, field of view=22 cm, flip angle=20°, number of excitations=1, 124 1.5-mm slices). Regions of interest approximated the saccadic circuitry, including the prefrontal cortex (containing the dorsolateral prefrontal cortex), premotor cortex (containing frontal eye fields, supplementary eye fields, anterior cingulate), sensorimotor cortex, and occipitoparietal cortex as well as the caudate, thalamus, and vermis, as described previously
(7,
8). Volumes were obtained by three raters (inter- and intrarater reliability >0.80) using stereological principles
(8).
Analyses of variance examined sex and group differences on antisaccade variables. Pearson correlations examined associations with age in each group. Analyses of covariance investigated sex and group volume differences in the regions of interest with cerebral volume entered as a covariate. Multiple regression models predicted antisaccade variables from the regions of interest in each group. Cerebral volume, which did not differ significantly between groups, was entered as covariate (forced entry). Regions of interest were entered as predictors (stepwise method, probability to enter p=0.05). Regressions were rerun in patients, with Positive and Negative Syndrome Scale scores as predictors in addition to the regions of interest.
Results
Male and female subjects differed in terms of amplitude gain (mean=–93.70 [SD=18.26] versus –107.75 [SD=11.63], respectively) (F=4.99, df=1, 38, p=0.03) and tended to differ in terms of latency (mean=302.90 [SD=86.96] versus 356.15 [SD=129.06]) (F=3.29, df=1, 38, p=0.08) but not error rate. There were no group-by-sex interactions for antisaccade or region of interest variables. Sensorimotor cortex volume was larger in female subjects (mean=126.00, SD=13.20) than in male subjects (mean=118.69, SD=16.76) (F=4.95, df=1, 38, p=0.03). Therefore, sex was entered as a covariate into analyses involving gain, latency, or sensorimotor cortex. Age was not significantly correlated with antisaccade or region of interest variables.
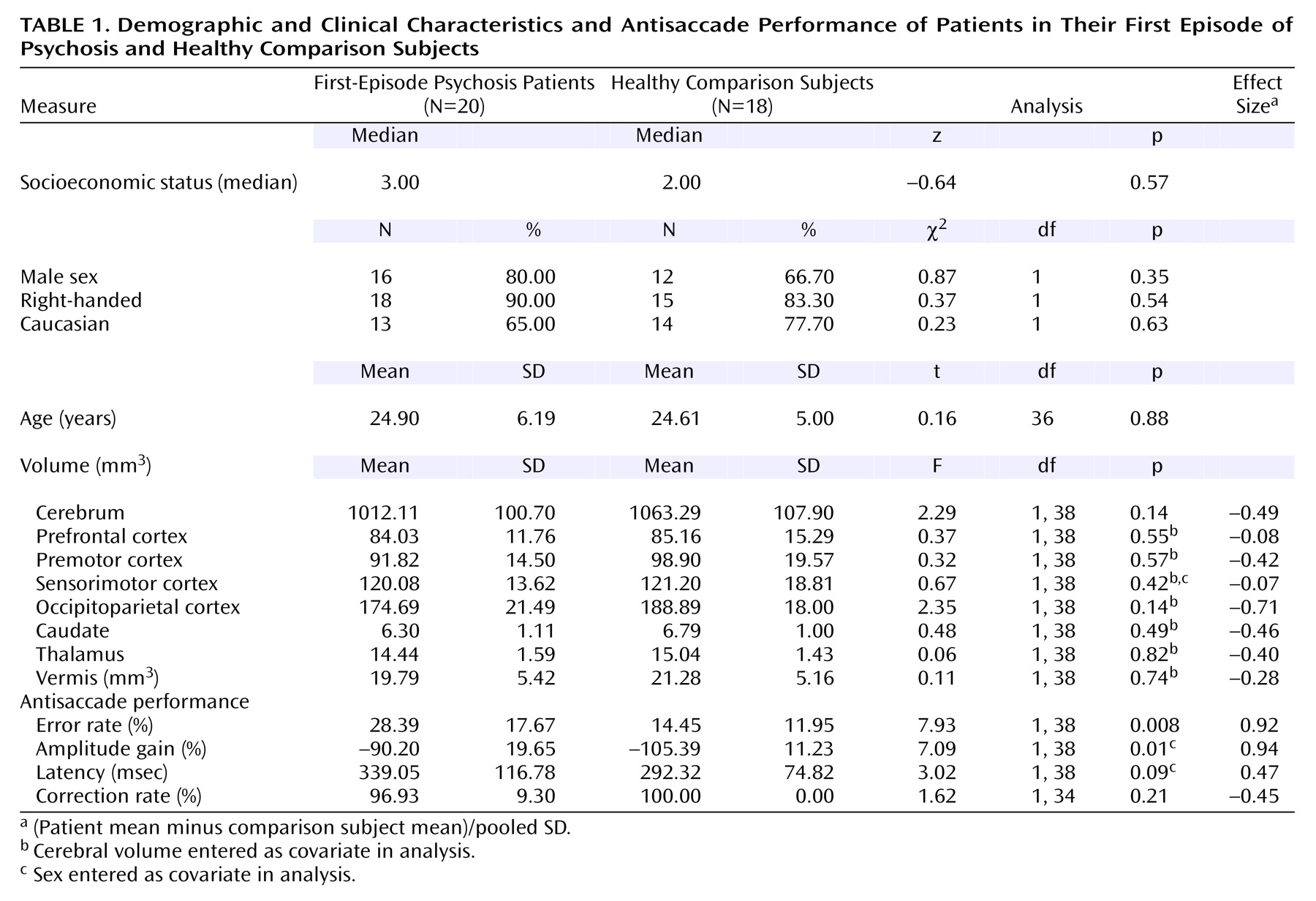
Group comparisons are summarized in
Table 1.
For the patients, cerebral volume and sex were entered as covariates into the initial multiple regression model predicting latency (R=0.41). The final model accepted caudate volume as a predictor (adjusted R2=0.23; R2 change=0.18; significance of change: p=0.05) and approached statistical significance (F=2.91, df=2, 17, p=0.07). Larger volumes predicted longer latencies (β=0.47). Cerebral volume and sex were entered as covariates into the initial multiple regression model predicting amplitude gain (R=0.46). The final model accepted caudate volume as a predictor (adjusted R2=0.33; R2 change=0.22; significance of change: p=0.02) and reached significance (F=4.15, df=3, 19, p=0.02). Larger volumes predicted hypometric gain (β=0.52). No region of interest volumes predicted error or correction rates. Adding symptoms to the model yielded negative symptoms as a predictor of error rate (adjusted R2=0.26; R2 change=0.34; significance of change: p=0.01) and reached significance (F=4.17, df=2, 16, p=0.04). Higher symptom scores predicted more errors (β=0.63). Symptoms did not predict amplitude gain, latency, or correction rate.
For the comparison subjects, cerebral volume was entered as a covariate into the initial multiple regression model predicting error rate (R=0.01). The final model accepted premotor cortex volume as a predictor (adjusted R2=0.27; R2 change=0.36; significance of change: p=0.01) and attained significance (F=4.18, df=2, 15, p=0.04). Larger volumes predicted fewer errors (β=–0.69). No region of interest volumes predicted amplitude gain or latency.
The lack of relationship between sensorimotor cortex volume and antisaccade error rate in either group was not affected when sex was entered into the analyses as a covariate.
Since antipsychotics can affect caudate volume
(9), and because of the observed relationship between caudate volume and both amplitude gain and latency, we compared the healthy subjects with the patients grouped by treatment (none, typical antipsychotics, atypical antipsychotics). There were no differences in caudate volume with cerebrum as a covariate (F=0.25, df=3, 38, p=0.86). The groups differed in terms of latency after sex was entered as a covariate (F=2.95, df=3, 38, p=0.05); Bonferroni’s t tests showed differences between comparison subjects (mean=292.32, SD=74.82) and patients treated with typical antipsychotics (mean=409.97, SD=143.62) (p=0.05) but not those receiving atypical antipsychotics (mean=288.92, SD=33.40) or no treatment (mean=306.18, SD=98.13). The groups tended to differ in terms of amplitude gain after sex was entered as a covariate (F=2.59, df=3, 38, p=0.07), with differences between comparison subjects (mean=–105.39, SD=11.23) and patients receiving atypical antipsychotics (mean=–81.45, SD=7.56) (p=0.07) but not those receiving typical antipsychotics (mean=–91.66, SD=22.87) or no treatment (mean=–92.95, SD=21.33).
Discussion
First-episode psychosis patients in this study displayed increased antisaccade error rate, reduced amplitude gain, and a tendency toward prolonged latency
(10).
Error rate was correlated with premotor cortex volume among comparison subjects but not among patients. This lack of relationship was unlikely due to sample size, since the patient group was larger than the comparison group. It is possible that the early-stage disease process, which led to increased errors but not significantly reduced volumes, altered this relationship among patients
(6).
Among patients, negative symptoms predicted antisaccade errors. This observation is compatible with studies relating negative symptoms to the frontal lobe
(11). Caudate volume predicted latency and gain, consistent with observations of the striatum in saccadic disinhibition
(2,
4). The reason why larger caudate volumes predicted longer latencies and reduced gain could be antipsychotic treatment, since both antisaccade variables differed between groups of untreated patients, comparison subjects, and patients receiving typical and atypical antipsychotics.
The regions of interest studied here do not conclusively demonstrate which specific subarea of the premotor cortex relates to antisaccade errors in healthy individuals. We also cannot rule out that other volumes, such as that of the dorsolateral prefrontal cortex, might relate to performance. Finally, limitations in region of interest segmentation might also have prevented us from detecting cortical correlates of gain and latency.