The variables that determine the extent of clinical improvement with drug treatment of psychosis remain largely elusive, although genetic factors very likely contribute to the unexplained individual variation in treatment response. The search for genetic factors that influence response to antipsychotic drugs has focused primarily on polymorphisms in candidate genes associated with pharmacological mechanisms, most notably the serotonin 5-HT
2A and dopamine D
2 -like receptors
(1) .
It is well established that some symptom clusters in schizophrenia, such as negative features and cognitive dysfunction, do not respond to pharmacotherapy as well as, or in parallel with, the positive symptoms. Few pharmacogenetic studies have addressed this differential response, although 5-HT
2A and 5-HT
2C gene associations with negative but not positive symptom response have been reported
(2,
3), and dopamine D
2 (4) and D
3 (3) receptor polymorphisms are reportedly associated with improvement in positive symptoms. These are generally small effects, and much of the variance in response remains unexplained. In many studies, variability in previous treatment with antipsychotic drugs introduces another confound.
The 5-HT
1A receptor is another candidate gene that may influence antipsychotic drug response, particularly in relation to the negative and cognitive features of schizophrenia
(5) . This receptor is also strongly implicated in antidepressant drug action. A common genetic polymorphism (–1019C/G) in the promoter region of the 5-HT
1A receptor, which has been found to have functional effects on gene expression, is associated with depression and suicide
(6) . We investigated the association of this polymorphism with symptom response to initial antipsychotic drug treatment.
Method
We recruited 63 drug-naive Spanish Caucasian subjects (mean age=25.1 years, SD=6.5; 45 [71%] males) who presented with a first psychotic episode. None had a prior history of medication with antipsychotics, antidepressants, or mood stabilizers, and none had a comorbid DSM-IV diagnosis of substance abuse or dependence or any physical illness. The study protocol was approved by the local ethical committee, and all patients gave written informed consent. Study subjects were treated according to standard clinical practice; drug treatment was reviewed after approximately 6 weeks and modified as needed. At 3 months, most patients were taking risperidone (N=19) or olanzapine (N=18), and others were taking quetiapine (N=10), haloperidol (N=6), ziprasidone (N=4), amisulpride (N=1), or no antipsychotic (N=5). Of those taking antipsychotics, nine were also taking an antidepressant medication and five were on lithium; of those not taking antipsychotics, five were taking an antidepressant medication. The Positive and Negative Syndrome Scale (PANSS) and the Calgary Depression Scale were used to assess the patients’ symptoms at initiation of treatment and again 3 months later.
All genotyping was carried out blind to clinical status. Genomic DNA was extracted from whole blood samples by standard techniques. Genotypes for the –1019C/G promoter region polymorphism of the 5HT 1A receptor gene were identified following two-primer polymerase chain reaction in two tubes using allele-specific reverse primers (forward primer: 5′CTGAGGGAGTAAGGCTGGAC3′; reverse primers: 5′GAAGAAGACCGAGTGTGTCTTCC/G3′). The reaction product underwent electrophoresis on a 3.5% agarose gel, from which alleles were identified.
SPSS version 10.0 (SPSS, Inc., Chicago) was used to conduct a univariate analysis of variance (ANOVA) to investigate the effects of genotype on changes in PANSS and Calgary Depression Scale scores and to compute goodness of fit statistics (R 2 ). Data are expressed as means with error bars denoting SD.
Results
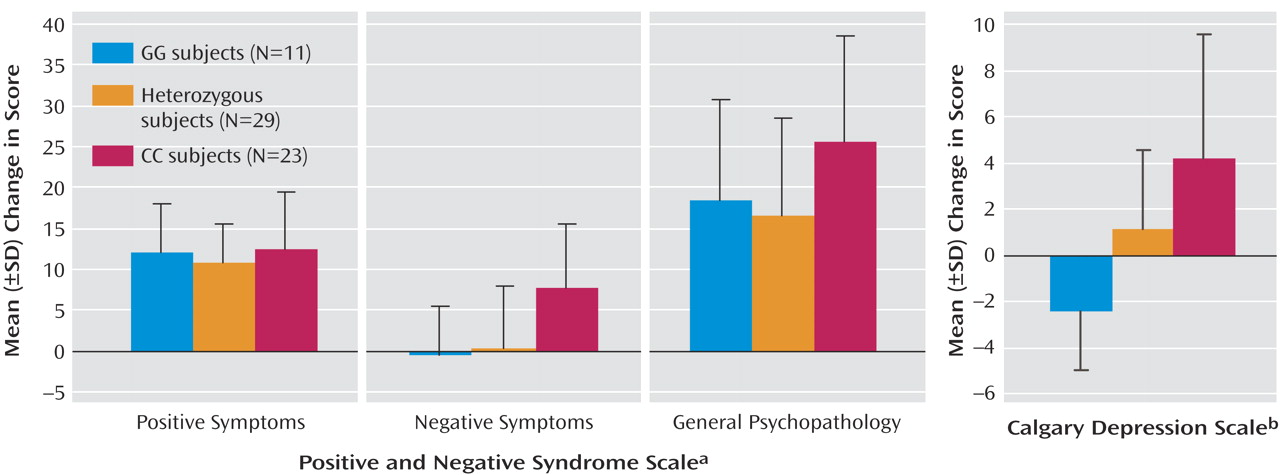
Eleven subjects had the GG genotype, 23 had the CC genotype, and 29 were heterozygous; genotype distribution was in Hardy-Weinberg equilibrium. Improvement in total PANSS score at 3 months was significantly associated with genotype (F=5.26, df=2, 60, p<0.01). As
Figure 1 shows, this result reflected a highly significant association with improvement in negative symptoms and an effect on changes in general psychopathology but not positive symptoms. Presence of the G allele was associated with, on average, no improvement in negative symptoms, whereas subjects with the CC genotype showed substantial improvement. Stepwise linear regression analysis indicated a significant effect of negative symptom subscale score before treatment on change in negative symptoms but no significant effect of age. An effect of genotype on this baseline negative symptom score was also identified (mean PANSS scores: GG=17.8, GC=15.7, CC=21.6; F=4.91, df=2, 60, p<0.02; R
2 =0.141), although after including this baseline score as a covariate, the effect on change in negative symptoms remained significant (F=3.95, df=2, 59, p<0.05; R
2 =0.421).
A strong association of genotype with change in Calgary Depression Scale score was also observed (
Figure 1 ). Stepwise linear regression analysis indicated no significant effect of age but an effect of Calgary Depression Scale score before treatment on the change in score. There was no significant association of genotype with this baseline depression score (F=2.18, df=2, 60, p>0.1); after including it as a covariate in the ANOVA, the strong association of genotype with change in the Calgary Depression Scale score remained (F=10.85, df=2, 59, p<0.001; R
2 =0.768). Subjects with a GG genotype demonstrated a significant worsening of depressive symptoms (one sample t test: t=–3.20, df=10, p<0.01). After 12 patients who had diagnoses of schizoaffective disorder, major depression, or bipolar disorder were excluded, the association of genotype with change in depressive symptoms remained (F=6.075, df=2, 48, p<0.01).
Sex introduced as a cofactor did not significantly affect the results in either of these analyses, demonstrating no significant main effect or sex-by-genotype interaction. Given the wide variety of drug treatments received by patients in this naturalistic study, it was not possible to conduct a rigorous analysis of possible differential effects of the various antipsychotics. However, in an analysis of patients receiving treatment with risperidone or olanzapine, a significant genetic association was demonstrated in both groups, with the G allele predicting poorer improvements in negative symptoms (risperidone: F=5.37, df=1, 17, p<0.05; olanzapine: F=10.66, df=1, 16, p<0.01) and in depression score (risperidone: F=9.29, df=1, 17, p<0.01; olanzapine: F=6.79, df=1, 16, p<0.02).
The changes in negative symptom subscale score and Calgary Depression Scale score in response to treatment are strongly correlated (Spearman’s rho=0.519, p<0.001). When change in Calgary Depression Scale score was introduced as a covariate in analyzing the effect of genotype on change in negative symptom score, the association remained significant (F=3.64, df=2, 59, p<0.05).
Discussion
These data show that a functional polymorphism of the 5-HT
1A receptor gene is strongly associated with changes in both negative features and depressive symptoms after 3 months of initial treatment of first-episode psychosis. The associations were independent of baseline scores, themselves strong predictors of response, indicating a direct effect of genotype on symptom response. The influence in each case is a particularly strong one, accounting for 21% and 25% of the variance in negative and depressive symptom change, respectively. After correction for baseline score, the predictive value of genotype in determining treatment response was approximately 42% for negative symptoms and 77% for depressive symptoms. Genotyping also identified a subgroup of patients (N=11) who experienced a worsening of depressive symptoms after antipsychotic treatment; these patients may be particularly liable to relapse
(7) .
Despite the strong correlation between negative and depressive symptom responses to treatment, the effect of genotype on negative symptom change is not solely explained by its influence on depressive symptoms, as demonstrated by the significant association found with change in negative symptoms while covarying for change in Calgary Depression Scale score.
The effect on affective symptoms was not due to a subgroup of subjects with primarily affective psychosis, since the association with depressive symptom response remained after this subgroup was excluded from the analysis, leaving only those with schizophrenia. The effect of genotype on negative and depressive symptom response to treatment also appears to transcend individual drug type; the associations remained true in groups of patients receiving either olanzapine or risperidone. Whether the effect extends to other atypical or conventional antipsychotics remains to be investigated.
The G allele of the –1019 polymorphism, which was associated with poorer response in our study subjects, is thought to bring about a loss of the control of gene expression by inhibitory transcription factors, theoretically resulting in increased 5-HT
1A autoreceptor expression and, consequently, reduced 5-HT transmission, predisposing for depression
(6) as well as anxiety and depression-related personality traits
(8) . Given the important role the 5-HT
1A receptor is thought to play in the action of some antidepressant drugs, it is notable that an association of the –1019C/G polymorphism with antidepressant response has been reported
(9) .
These findings would suggest that 5-HT
1A receptor expression or its regulation is important in mediating the response of negative and depressive symptoms to antipsychotic drug treatment. The two major drugs involved in this study, risperidone and olanzapine, do not have significant direct effects at the 5-HT
1A receptor but influence serotonin neurotransmission by other mechanisms, including antagonism of 5-HT
2A receptors. However, these two serotonin receptor subtypes are localized on the same neurons in, among other regions, the frontal cortex
(10), where they have opposing functional effects. The action of atypical antipsychotics on dopamine release in the prefrontal cortex has been proposed to contribute to their effects on negative and cognitive features of schizophrenia and is mediated by a combination of 5-HT
2A and weak D
2 receptor antagonism
(11) . This mechanism requires functionally active 5-HT
1A receptors
(12,
13) . Genetically determined differences in 5-HT
1A receptor density or function may thus influence antipsychotic action on cortical dopamine release and thereby on negative symptom response.
Whatever the mechanisms underlying the genetic associations observed here, these findings indicate the important role played by the serotonin system and the 5-HT 1A receptor in the treatment response of the negative and depressive symptoms of schizophrenia.