Second-generation, or atypical, antipsychotic medications have been used to treat psychiatric illness in children and adolescents with increasing frequency over the last decade
(1 –
4) . This is due in part to their ability to effectively control many symptoms associated with cognitive deficits, mood disorders, and difficulties with impulse control and excitability, with resultant functional recovery
(5) . These effects may be achieved with lower rates of extrapyramidal side effects and tardive dyskinesia than has been observed with use of “typical” antipsychotic agents. However, therapeutic success is associated with substantial weight gain, with resultant increased risk of developing insulin resistance syndromes, cardiovascular disease, and other complications of obesity in a population already prone to these comorbidities
(6 –
9) . Weight gain may itself negatively impact patient compliance, being a major reason for drug discontinuation in one recent drug comparison
(10) .
Understanding the mechanism of weight gain during treatment with atypicals may help in the design of effective treatments and preventive measures to address these changes. Hyperglycemic hyperinsulinemic clamp studies in normal subjects showed that acute treatment with olanzapine had no effect on insulin secretion
(11) . Subsequently, Henderson et al. showed that long-term treatment with atypical antipsychotics caused insulin resistance and decreased glucose effectiveness in lean patients with schizophrenia
(7) . Studies in dogs showed that β-cell compensation for atypical-induced hepatic insulin resistance was impaired when compared with that in animals with obesity induced by a high-fat diet, suggesting that neural input may influence the insulin response to these agents
(8) . Thus, atypicals may have direct effects on hypothalamic appetite centers, alter satiety signals emanating from adipose tissue or gut, or create hormonal resistance to satiety control.
Studies to determine safe and effective means of weight control for patients taking atypicals have been short term and involved small numbers of subjects, leading one reviewer to conclude that lifestyle change is the sole treatment currently available for these patients
(12) . While lifestyle change has been the most effective means of weight loss in obese adults
(13 –
16), it is particularly difficult to successfully institute behavior and dietary modifications in subjects with neuropsychiatric disorders
(17) . Metformin treatment has been utilized to treat obese adolescents with a family history of type 2 diabetes
(18) . The drug, which inhibits hepatic glucose production, was well tolerated and prevented continued weight gain while it decreased measures of insulin resistance
(18) . We found that metformin therapy also resulted in weight loss in a 12-week study of 19 subjects ages 10–18 who had gained weight during atypical antipsychotic therapy
(19) . Herein we confirm these results in a double-blind, placebo-controlled fashion at higher metformin doses over a longer treatment period.
Method
Subjects
Children ages 10–17 years who had gained more than 10% of their predrug weight during less than 12 months of treatment with a targeted atypical antipsychotic agent—olanzapine, risperidone, or quetiapine—were recruited for the metformin trial. Subjects could be taking other psychotropic agents, but only one atypical, whose dose had not changed by more than 25% over the past 3 months. Those prescribed other agents that may affect body weight (lithium, valproate, carbamazepine, topiramate, and other antidepressants) were required to have been on stable regimens of these drugs for at least 30 days prior to starting the study and to remain on these regimens, with stable doses, throughout the duration of the study. Subjects with previously diagnosed diabetes mellitus, seizure disorders, a history of neuroleptic malignant syndrome, mental retardation (IQ <50), or pregnancy were excluded. Baseline anthropometric measures and laboratory studies were performed only after signed parental informed consent was obtained, with signed child assent where possible. The successful collection of anthropometric measures constituted a complete study visit.
Subjects with consent who were found to have exclusion criteria either after baseline testing or during the study were classified as dropouts. Subjects were dropped from the study if the study drug was discontinued but not if another medication was added. They were also considered dropouts if they did not attend the final study visit but not if they failed to attend an interval visit. No subject who completed the study failed to attend more than one interval visit. The study protocol and informed consent document were found to follow the guidelines of the Health Insurance Portability and Accountability Act of 1996 and were approved by the Scientific Advisory Committee and the Institutional Review Board of the Clinical Research Center of the University of Cincinnati and Children’s Hospital Medical Center.
Study Protocol
A randomized, double-blind, placebo-controlled design was used; the study drug was metformin. Subjects were given one 500-mg capsule containing either metformin or an identical-appearing placebo at their evening meal for 1 week, after which a second dose was added before breakfast. After the second week, the dose was increased to 850 mg given with these meals for an additional 14 weeks. Subjects visited the Clinical Research Center at baseline and weeks 4, 8, 12, and 16 for anthropometric measurements and were interviewed for side effects or adverse events. Safety and study laboratory determinations were obtained as will be described in a following section. Anthropometric measures, which included height, weight, and waist circumferences, as well as laboratory evaluations, were performed in the fasting state in accordance with the National Growth and Health Study protocol of the National Heart, Lung, and Blood Institute
(20) .
The same registered dietician, blinded to treatment group, gave nutritional counseling at baseline and weeks 4, 8, and 12. No specific diet was assigned, but after initial assessment of the subjects’ usual eating patterns and exercise habits, the dietician helped determine individualized areas for improvement and set goals for small changes. Each subject was given the “Healthy Food Choices” fold-out meal-planning tool (American Dietetic Association, Chicago).
The principal investigator (D.J.K.) and study coordinator were blinded to group assignment and to the study data after their collection at each visit. The study statistician (B.A.B.) supervised data entry, interim analysis, and results of safety laboratory tests and regularly communicated interim findings to a data safety monitoring board. The principal investigator was given access to data in a blinded fashion if necessary for patient safety. Care was taken to have the study investigators remain blinded during subject hospitalizations by having the subjects receive “study drug.” The Clinical Research Center was one floor below the inpatient service, facilitating study visits.
After completion of the study, the principal investigator, still blinded to treatment group, reviewed patient data to determine whether oral glucose tolerance testing (GTT) was warranted. The criteria included obesity (body mass index >95th percentile for age) or excessive weight gain during the study (gain >10% of baseline weight) associated with a fasting insulin value over 20 μU/ml and/or a glucose level over 95 mg/dl. Body mass index was calculated as weight (in kilograms) divided by height (in meters) squared.
Power calculations were performed by using data from our earlier patient series of 19 subjects, who had a mean weight loss of 2.9 kg (SD=3.1 kg)
(19), and from pilot data on 16-week weight gain after initiation of treatment with atypicals (mean=4.0 kg, SD=4.1). If an alpha of 0.05 in a two-sided test and power of 0.90 are assumed, nine children per treatment group would be needed. With such a low number, however, a single unexpected extreme value would have a major effect on the results of the study, so we elected to double the number of subjects to 20 in each group.
Laboratory Studies
At each visit, blood samples were obtained for “safety” determinations of serum lactic acid, sodium, potassium, BUN, creatinine, bicarbonate, and liver enzymes and for serum pregnancy testing. Fasting serum insulin and glucose levels were measured at baseline and at weeks 8 and 16, as previously described
(21) . Homeostasis model assessment (HOMA-IR), which correlates with estimates of insulin resistance measured by the euglycemic clamp technique, was used as an index of insulin resistance
(22) . The guidelines of the American Diabetes Association define a fasting glucose level of 100 mg/dl or higher as impaired fasting glucose and a level of 126 mg/dl or higher as diabetes
(23) .
Statistical Analysis
Descriptive statistics were calculated in the standard way. To compare race and other baseline categorical variables in the two treatment groups, a chi-square test was used with 1 degree of freedom. To compare continuous variables at baseline for the two treatments, we used an analysis of variance with least squares means. Student’s t tests were employed to compare the changes from baseline to week 4, week 8, week 12, and week 16 in anthropometric measures. If the variances were unequal, we used the Satterthwaite adjustment. We used general linear models to assess the treatment effect on the change in anthropometric measure, adjusting for other factors such as race, sex, or baseline measure. Because the study was conducted in growing children, age-adjusted z scores for weight and body mass index were calculated by using the program gc-calculate-BIV.sas (available from the Centers for Disease Control and Prevention [CDC]; http://www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm). This program uses the 2000 CDC Growth Charts, which are based on data from the National Health Examination Surveys and the National Health and Nutrition Examination Surveys
(24) . A longitudinal model was fit by using a mixed model to adjust for the inherent correlation among measures for the same patient. The mixed model was fit by using Proc Mixed in SAS version 9 (SAS Institute, Cary, N.C.) with an unstructured correlation structure and random intercepts for each patient. The model was also fit by assuming a common intercept for each subject, and the two results were compared. Goodness of fit was established by using Akaike’s information criterion and Bayesian information criterion. The significance of the parameter estimates was calculated by dividing the parameter estimate by the standard error of the estimate, resulting in a t statistic, which was compared to the standard t distribution with degrees of freedom determined by the Kenward-Roger method. All analyses were performed by using SAS version 9.
One can analyze clinical trial data on dropouts by carrying forward data to analyses of later or final visits or by using data on only the subjects who attend the later visits. Excluding data from subjects who dropped out because of diabetes or weight gain might have selectively eliminated the very subjects who failed to respond to the intervention by developing insulin resistance. To control for this possible bias, we analyzed the data by both methods. Because of the small number of subjects, the analyses reported herein were based on the assumption that dropouts were noninformative (see Results) and that the use of modeling procedures such as pattern mixture models or even a simple logistic model (with the binary outcome of dropout) would have little power to detect a pattern.
Results
Baseline Characteristics of Study Participants
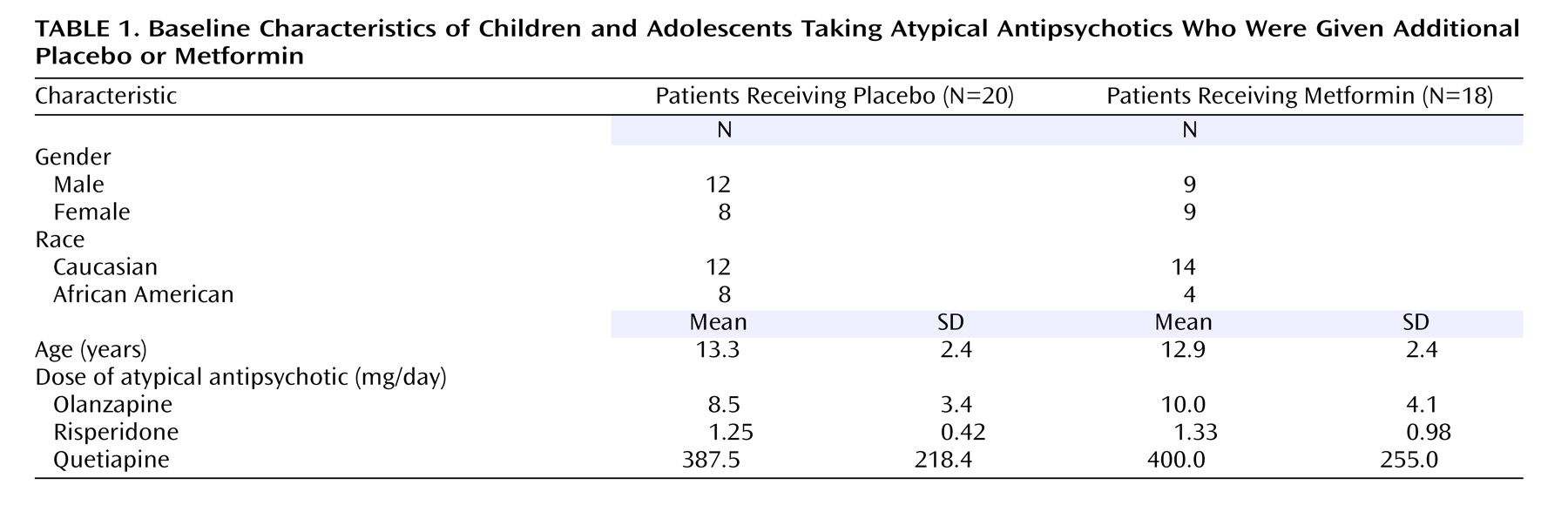
Thirty-nine subjects enrolled in the trial. One subject completed the informed consent process but developed acute psychosis before the baseline measurements were obtained and was therefore excluded from the analysis. Of the remaining 38, 18 were randomly assigned to active drug and 20 were assigned to placebo (
Table 1,
Figure 1 ). The subjects’ ages ranged from 10 to 17 years. There was no significant difference in the age or gender distribution of the subjects assigned to placebo and metformin treatment (
Table 1 ). Twelve African American subjects were enrolled in the trial: eight were randomly assigned to placebo treatment, while four were treated with metformin (p=0.28).
Eleven participants were receiving olanzapine, 14 risperidone, and 14 quetiapine. Neither the atypical nor the length of treatment with that agent before study entry differed between treatment groups; the mean duration of treatment was 8.0 months (SD=5.8) for the metformin group and 5.7 months (SD=4.2) for the placebo group. In addition, there were no group differences in the atypical dose (
Table 1 ).
Although all subjects were treated with a single atypical, each subject was receiving multiple psychotropic agents. There was no difference in number of drugs between treatment groups; the mean number for metformin was 2.9 drugs/subject (SD=1.6), and for placebo it was 3.3 drugs/subject (SD=1.1). The referring physicians frequently listed multiple psychiatric diagnoses, with the most common being bipolar disorder (N=20) and attentional disorders (N=17). Others were schizophrenia (N=4), oppositional defiant disorder (N=7), autism and Asperger’s syndrome (N=5), Tourette’s syndrome (N=2), schizoaffective disorder (N=1), and depression (N=4).
The mean weight gain after initiation of atypical therapy and prior to study entry was 9.4 kg (SD=7.5) in the metformin group and 10.8 kg (SD=6.8) in the placebo group (p>0.20). There was no difference in the mean age-specific percentile for body mass index at baseline, which was 88.3% (SD=16.2) for the placebo group and 87.8% (SD=12.8) for the metformin group. Nine placebo-treated and eight metformin-treated subjects had baseline body mass indexes above the 95th percentile for their age (categorized as obese).
Study Discontinuation
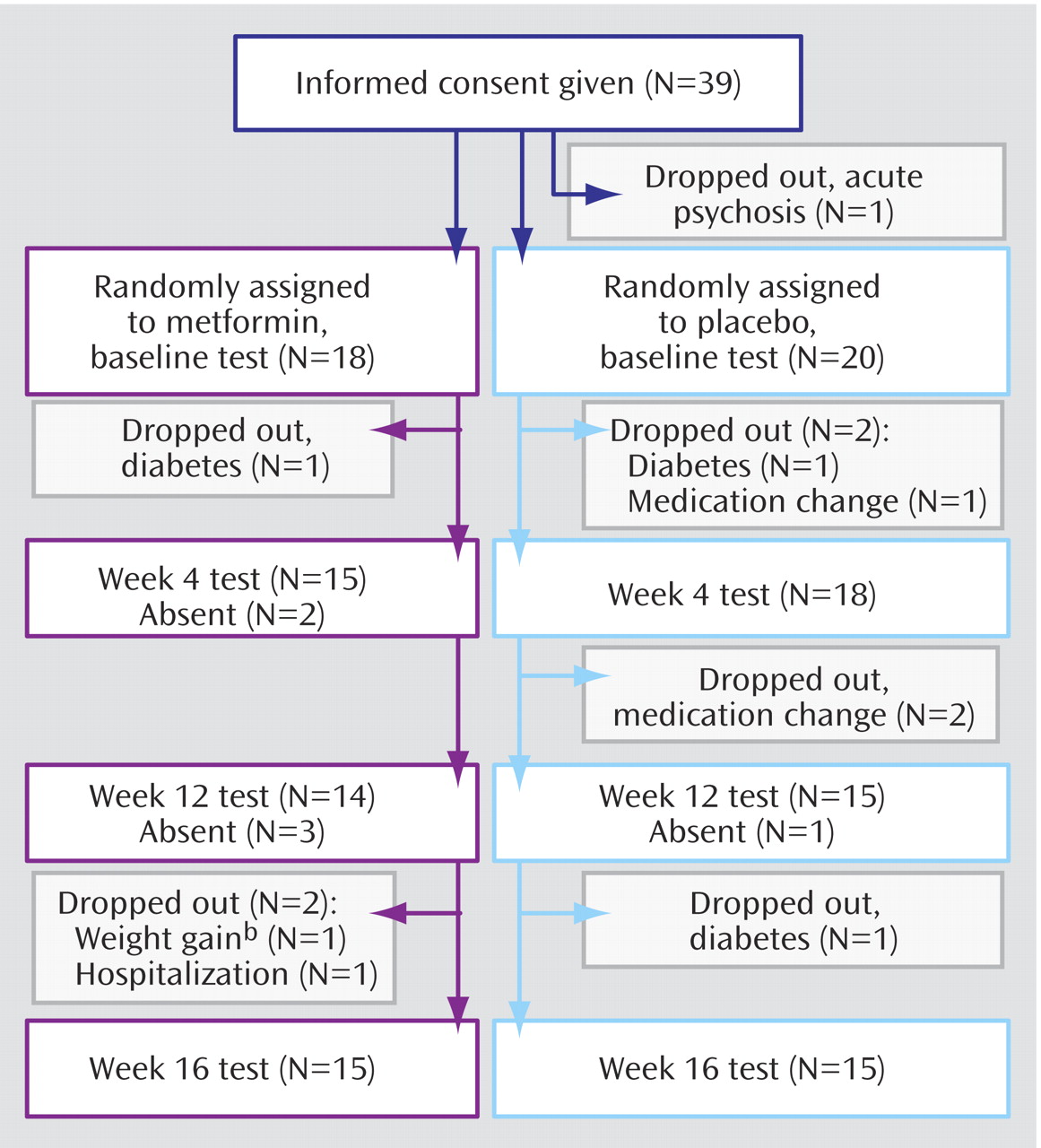
Study subject retention and dropouts are illustrated in
Figure 1 : eight randomly assigned subjects were considered dropouts because they discontinued the study before the week 16 visit. In the metformin group, three subjects dropped out: one was diagnosed with diabetes at baseline (elevated fasting glucose and insulin values), and two dropped out after the week 12 visit (one was hospitalized for a medication change; the other dropped out because of continued weight gain and was found from pill counts to be noncompliant). In the placebo group, five subjects dropped out: two because of diabetes (one case was diagnosed at baseline and included elevated fasting glucose and insulin values, and the other case was diagnosed after the week 12 study visit) and three because their psychiatrists elected to change the atypical antipsychotic (after data collection at baseline, week 4, and week 8, respectively). Thus, 15 subjects from each group completed the study by attending the final (week 16) visit. There was no statistically significant difference between the numbers of dropouts in the two treatment groups (p=0.70).
Six subjects required hospitalization for psychiatric illness, two from each atypical therapy group. Five hospitalized subjects were from the placebo group, and one was taking metformin (p=0.13). Only one subject missed a study visit as a result of hospitalization (
Figure 1 ). No serious adverse events resulted from metformin treatment, and there were no abnormal results from the safety laboratory determinations throughout the study (see Method section). The percentage of subjects reporting adverse events did not differ significantly between the placebo group (14 of 20) and metformin group (12 of 18) (p>0.90). Moreover, the numbers of events reported by the metformin subjects (32 adverse events) and the placebo subjects (34 adverse events) were similar. No significant difference was found in the reported occurrences of diarrhea, often cited as being associated with metformin.
Anthropometric Changes During Treatment
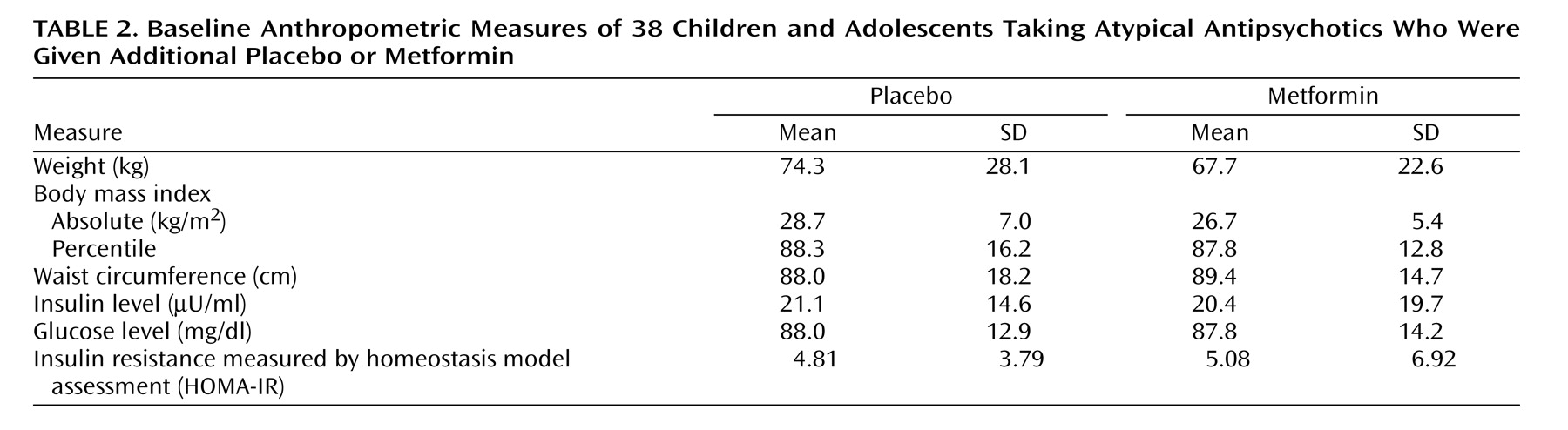
There were no statistically significant group differences in baseline anthropometric measures (
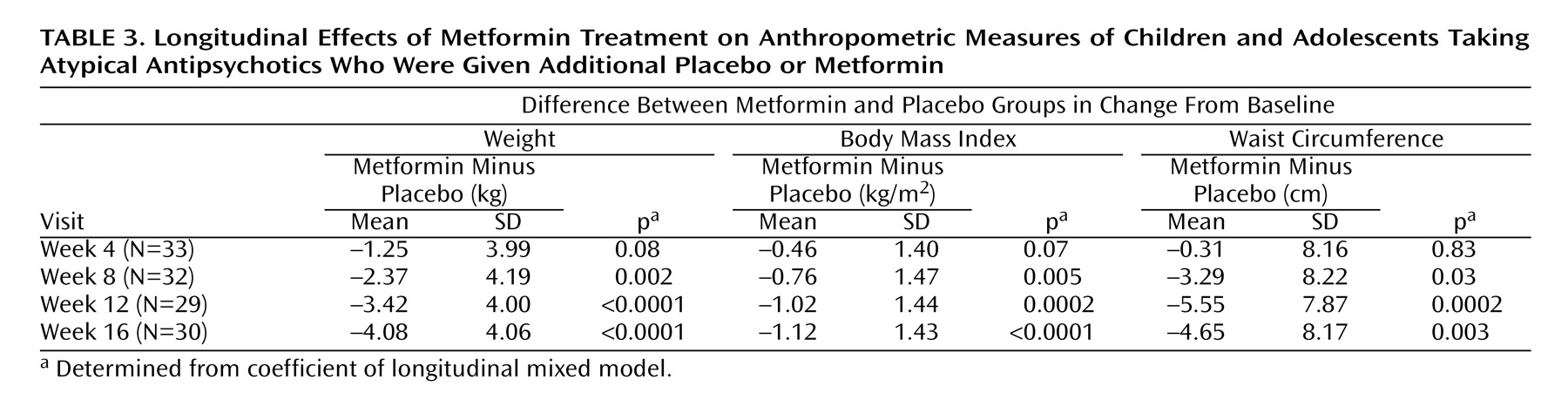
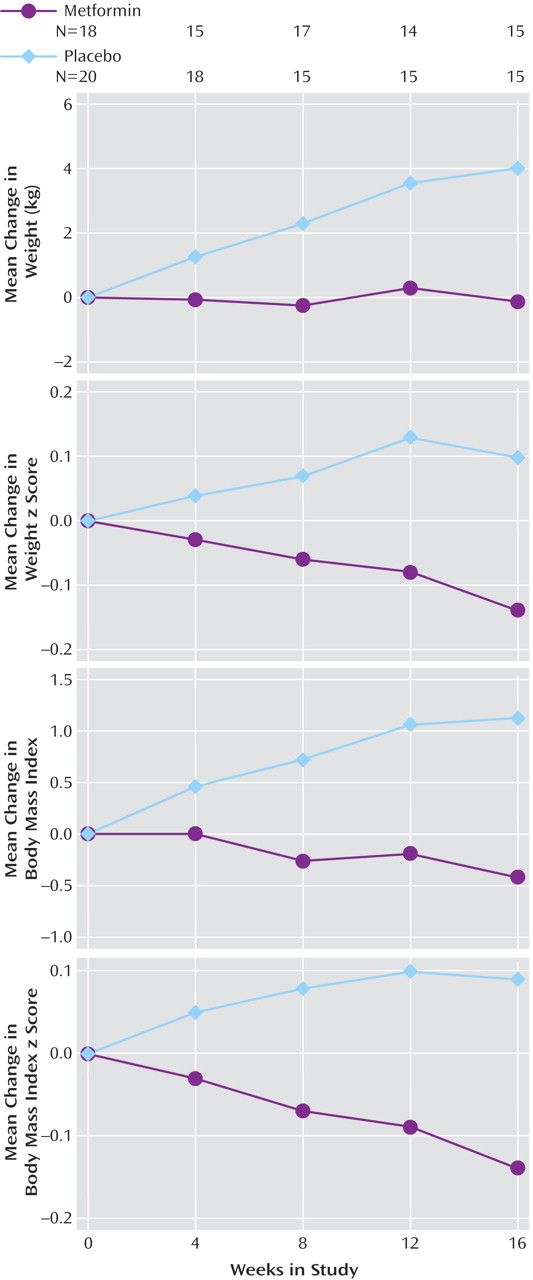
Table 2 ). The major study objective was to determine whether or not metformin treatment was more effective than placebo in preventing further weight accretion with atypicals. Drug treatment had a significant effect on weight gain as well as measures of obesity (body mass index) and weight distribution (waist circumference) over the course of the study (
Table 3,
Figure 2 ).
While the subjects given dietary instruction and placebo continued to gain weight (mean=4.01 kg, SD=6.23) over 16 weeks, the weight of the subjects treated with metformin showed little change over the treatment period (mean= –0.13 kg, SD=2.88), as shown at the top of
Figure 2 . Differences between the mean values were significant for all time periods and remained significant when baseline weight, body mass index, sex, and race were included in the statistical model. The rate of weight gain with placebo was continuous during all time intervals, with weights increasing at a mean rate of 0.31 kg (SD=0.44) per week (approximately 11 oz per week), while showing little change (mean change of –0.03 kg/week, SD=0.33) in the metformin-treated subjects. Similarly, the mean body mass index decreased by 0.43 (SD=1.07) in the metformin group at the same time it increased by a mean of 1.12 (SD=2.02) in the subjects taking placebo, a difference that was statistically significantly different at all time points (
Figure 2, third graph).
Because the study was conducted in growing children, the age-corrected changes in the standard deviations for both weight and body mass index (z scores) were determined. The z score for weight increased by 0.10 (SD=0.29) in the placebo group, and the final z score for mean weight was 1.72 (SD=0.99) above the mean for their age (
Figure 2, second graph). In contrast, the metformin group had a z score for mean weight that decreased by 0.14 (SD=0.21). The difference between treatment groups in the z score for mean weight increased gradually during the study, with the metformin group’s z score being 0.08 lower at 4 weeks (t=2.24, df=35, p=0.03), 0.14 lower at 8 weeks (t=2.48, df=30, p=0.02), and 0.24 lower at 16 weeks (t=2.58, df=28, p=0.02). The z score for the body mass index of the metformin-treated subjects decreased by 0.14 (SD=0.20) during the study (
Figure 2, bottom graph). In contrast, it increased by 0.09 (SD=0.25) in the subjects taking placebo (t=2.63, df=28, p=0.02). That metformin treatment also affected weight distribution was evident from measurements of waist circumference: that of subjects taking placebo increased by 3.64 cm (SD=6.91) over the course of the study while it decreased by 2.51 cm (SD=5.46) in subjects taking metformin. These changes were significant according to t tests at week 8 (t=2.67, df=28, p=0.02), week 12 (t=2.52, df=24, p=0.02), and week 16 (t=2.57, df=25, p=0.02) but not at week 4 (p=0.20).
Longitudinal analysis was used to verify the effects of treatment group and determine the importance of visit (or length of treatment) on weight accretion, body mass index, and waist circumference (
Table 3 ). Results of these analyses, carried out by using either raw patient data (not shown) or the change in the anthropometric measures (
Table 3 ), were not significant at the week 4 visit but became increasingly statistically significant over time, indicating a progressive effect of treatment group on anthropometric measures. The global test for significance was highly significant for each of the measures analyzed.
There were no differences in significance when the anthropometry data were analyzed by using the last value carried forward for analysis. When the longitudinal model generated with the raw patient data was fit by using a common intercept, the fit was substantially worse than with a random intercept owing to the varying anthropometric measures at baseline. Thus, only the results from the random-intercepts fit are reported here.
Changes in Measures of Insulin Sensitivity
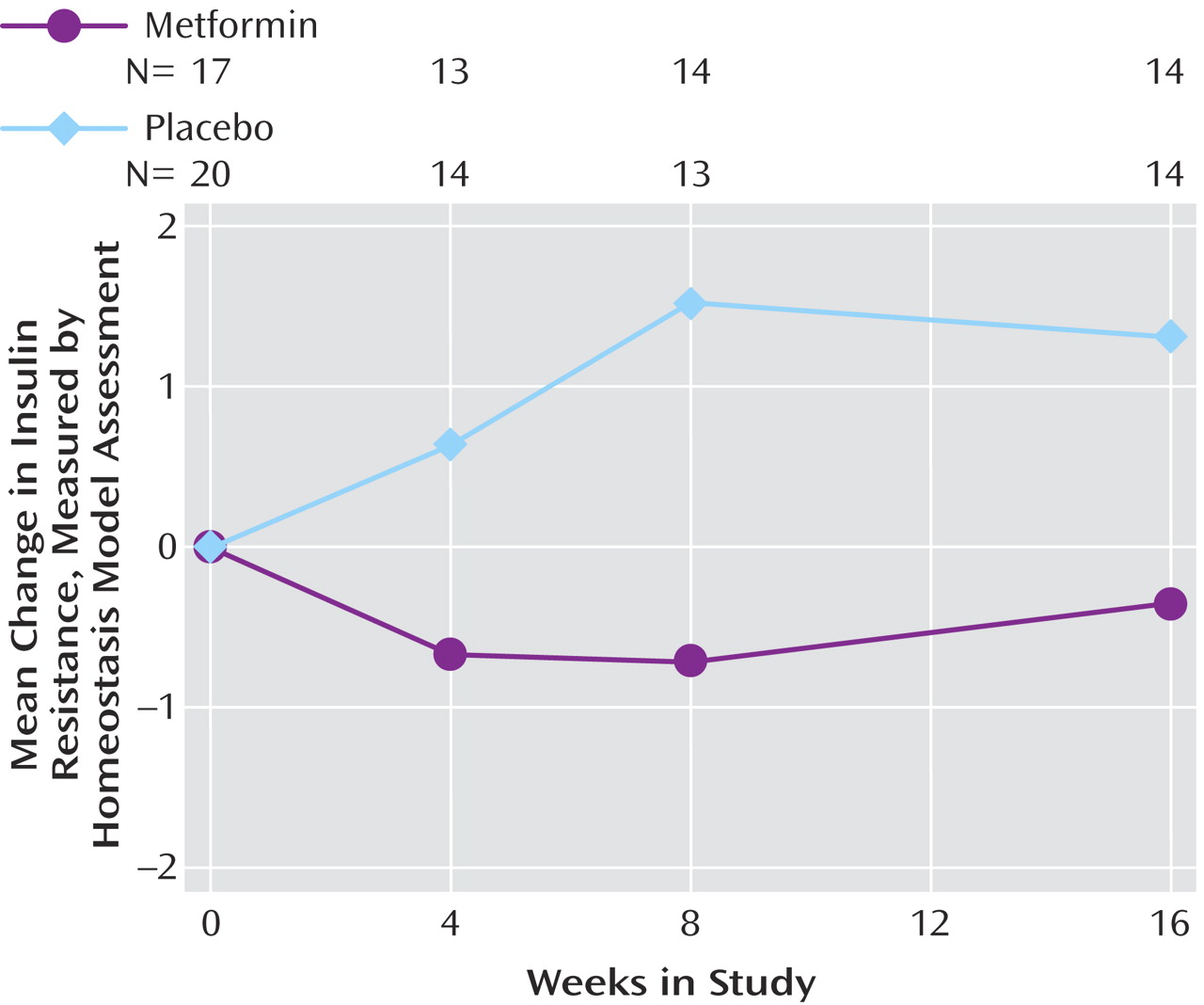
The baseline HOMA-IR values did not differ between treatment groups (
Table 2 ). During the first 8 weeks of follow-up, the mean value increased by 1.52 (SD=3.07) in the placebo group while it decreased by 0.71 (SD=1.20) in metformin-treated subjects (
Figure 3 ). Differences remained at the week 16 visit but were no longer statistically significant (p=0.17). As with the anthropometric data, there were only minor changes in the significance of the HOMA-IR data when analyzed by using the last value carried forward (p=0.16 for difference between treatment groups at week 16), and the model with a common intercept yielded a worse fit than that prepared by using random intercepts.
In addition to its effects on insulin sensitivity, metformin treatment was associated with a decrease in indications for performing a GTT at the end of the study. Testing, based on the criteria outlined in Method, was recommended for 12 subjects, 10 taking placebo and two treated with metformin (χ 2 =6.63, df=1, p<0.01). Of the metformin-treated subjects recommended for GTT, one was independently judged to be noncompliant on the basis of the pill count. The other refused testing because she had lost weight during treatment and had fasting insulin and glucose levels that had decreased from baseline to week 16 and were only borderline abnormal (insulin decreased from 22.9 to 16.9 μU/ml, and glucose decreased from 96 to 90 mg/dl). The GTT revealed impaired glucose tolerance in three placebo-treated subjects (two taking quetiapine and one taking olanzapine). In addition, two subjects who refused GTT had evidence of glucose intolerance (one with impaired fasting glucose at the final study visit and the other a previously mentioned placebo subject who developed diabetes after study completion). Both were receiving olanzapine therapy. Thus, in addition to the four study subjects identified with overt diabetes, four had early signs of glucose intolerance, and another eight participants showed evidence of insulin resistance. Metformin not only significantly abrogated the need for a GTT but also had an impact on the development of abnormal glucose tolerance.
Discussion
Weight gain has been a barrier to compliance with the administration of atypical antipsychotics, medications that effectively allay many of the symptoms of psychiatric illness in both adults and children. This side effect has been associated with increased risk for future cardiovascular disease and other complications of obesity, including the development of insulin resistance and overt diabetes. Therefore, clinical trials of medications that may affect weight gain have been conducted. One 12-week double-blind, placebo-controlled trial of sibutramine (which, like atypicals, interacts with serotonin 2c receptors) resulted in greater weight loss than dietary counseling and placebo treatment alone
(25) . Study subjects included patients taking atypicals for schizophrenia or schizoaffective disorder who were obese or overweight with cardiovascular risk factors. Despite modest weight loss with sibutramine, there was no improvement in insulin sensitivity or blood pressure, two important determinants of cardiovascular risk
(16) . In addition, anticholinergic and other side effects could limit the use of sibutramine in the psychiatric population. Similarly, amantadine induced weight stabilization in a 12-week study performed in 21 adults, without improvement in insulin values
(26) . However, its dopaminergic properties may result in worsening psychosis. Topiramate treatment has been reported to have some success in small, uncontrolled trials, but serious side effects also accompanied its use
(27) . In contrast, metformin treatment has been employed safely and effectively in the treatment of otherwise healthy obese adolescents
(18) and in a small open-label trial of obese adolescents and youth who had gained weight while taking atypicals
(19) .
In this study a randomized, double-blind, placebo-controlled design was used to determine whether or not metformin treatment could effectively prevent further weight accretion or cause weight loss in 10–17-year-old subjects who had experienced substantial weight gain in the first year of treatment with atypicals. Psychiatric subjects recruited for the trial were being treated with one of three atypicals (risperidone, quetiapine, and olanzapine) for varied diagnoses, including bipolar disorder, attentional and oppositional disorders, and schizophrenia. All subjects were taking other psychotropic medications (approximately three per subject).
Children treated with placebo continued to gain weight at a rate of 0.31 kg (approximately 11 oz) per week, despite three family sessions of dietary counseling. Weight stabilization was associated with metformin treatment and occurred in the context of an increase in height in growing children. Therefore, those receiving active drug experienced decreases in z scores for weight and body mass index. In addition, waist circumference decreased in metformin-treated subjects, indicating a decrease in visceral fat content and risk for diabetes and cardiovascular disease.
The relevance of metformin therapy as a treatment for weight gain induced by atypical antipsychotics was evident from the high incidence of insulin resistance and its consequences identified in study subjects. Diabetes was diagnosed in two study subjects as a result of baseline laboratory studies (both subjects had high fasting insulin and glucose values). Two placebo-treated subjects developed diabetes during the study or shortly after completion. Metformin treatment decreased insulin resistance, as indicated by HOMA-IR, which reflects insulin sensitivity. This drug effect reached its maximum at week 8 of the study, after which two subjects taking metformin who had high baseline insulin values and body mass indexes became noncompliant with treatment and one placebo-treated subject was diagnosed with diabetes. The impact of metformin on insulin sensitivity was also apparent from its significant effect on the need for follow-up GTT at the end of the study. Nearly all of the subjects recommended for this procedure (10 of 12) were from the placebo group. Three subjects who had GTT showed impaired glucose tolerance, and two who were not tested had evidence of glucose intolerance (one was later diagnosed with diabetes, and the other had impaired fasting glucose at the last study visit). The psychiatric drug taken by the subject had no apparent impact on the need for GTT (the 12 subjects were evenly distributed among the three atypicals) or the development of abnormalities of glucose tolerance, including overt diabetes (two subjects diagnosed with diabetes were taking olanzapine and two were taking quetiapine). However, the study was not powered to determine the significance of each atypical for any of the study outcome measures. These studies show that metformin therapy abrogates abnormalities in glucose tolerance resulting from atypical treatment by decreasing insulin resistance. While the statistical analyses presented herein for both anthropometric measures and HOMA-IR were performed by using data collected at each visit, there was no change in their significance in separate analyses performed when the data were carried forward to subsequent visits (not shown).
Lifestyle modifications have been applied successfully for weight control in several population-based studies in which predominantly self-referred, highly motivated subjects at risk for disease progression were assisted in weight reduction programs by frequent contact with investigators
(13) . The present study was not designed to determine whether or not addition of a significant lifestyle intervention to metformin treatment would improve drug response. Subjects were enrolled because of percentage weight gain and not because of obesity or overweight with cardiovascular risk (85% were obese at baseline). Nor can it be stated that subjects enrolled because of a strong personal motivation to lose weight, since they were referred by their physicians and enrolled by parents. Diet instruction was given and reinforced on two other occasions during the study, but no protocol for lifestyle change was offered and only small changes in diet and exercise were recommended. It has been stated that subjects with neuropsychiatric disorders (including subjects enrolled in this study) are less likely to succeed with lifestyle interventions alone than are those without mental illness
(17) . Indeed, it is not known whether or not addition of lifestyle modifications is a feasible approach to obesity in this population. Therefore, it can be stated that medication therapy alone results in decreased weight accretion and insulin resistance in patients receiving atypicals.
This study verifies that metformin treatment is well tolerated in children and adolescents. No adverse events could be attributed to the study drug in this short-term trial. There were no significant differences in side effects or dropout rate attributable to metformin treatment. Indeed, the dropout rate was higher in the placebo-treated subjects (but without statistical significance). While participants in this study did not all have the same psychiatric diagnosis and did not all take the same atypical agent, the study group was representative of the patients served in most outpatient psychiatric settings.
In conclusion, this placebo-controlled, double-blind study indicates that metformin is safe and effective in treating the weight gain and insulin resistance that develop in a substantial number of children and adolescents treated with atypical antipsychotics. Future investigations must focus on the use of metformin to treat weight gain occurring at the outset of atypical treatment. In these investigations, it will be important to determine risk factors associated with weight gain with atypicals and whether or not risk for weight gain and treatment success is linked to the psychiatric diagnosis. Another shortcoming of the present study was that a single dose of metformin was used in a group having varied body composition. In addition, it was difficult for the study subjects to remain compliant with their assigned treatment plan, despite the short duration of the study and the positive effects of treatment. Frequent problems in compliance with medication administration have been reported in other studies of psychiatric subjects
(10) . Lifestyle interventions coincident with optimization of psychiatric care and medication management may have an impact on this factor. While it is likely that compliance difficulties will always be present in this patient population, even with increased supervision, alterations in drug delivery with the addition of agents that allay significant side effects may improve patient adherence to treatment and treatment outcome.