Increasing evidence supports maternal exposure to several infections as risk factors for schizophrenia
(1 –
3) . Prenatal and perinatal exposure to herpes simplex virus type 2 is a known cause of congenital brain anomalies
(4) . In a previous investigation from the Collaborative Perinatal Project, maternal immunoglobulin (IgG) antibody levels to herpes simplex virus type 2 were associated with psychosis in offspring
(5) . Although the results were compelling, there were a modest number of cases (N=27), a broad definition of psychosis, and an interpretation of IgG optical density measures that differed from standard approaches.
We examined maternal herpes simplex virus type 2 in a considerably larger sample of offspring restricted to schizophrenia and other schizophrenia spectrum disorders (mostly schizophrenia or schizoaffective disorder). We used a standard approach to assay archived prenatal serum specimens to quantify herpes simplex virus type 2 seropositivity and IgG antibody. In exploratory analyses, we similarly examined herpes simplex virus type 1 and cytomegalovirus.
Method
The present investigation was based on the Prenatal Determinants of Schizophrenia study, described previously
(6) . Briefly, the cohort was enrolled in the Child Health and Development Study, which recruited nearly every pregnant woman under obstetric care from the Kaiser Permanente Medical Care Plan in Alameda County, Calif., between 1959 and 1966. With the Prenatal Determinants of Schizophrenia Study
(6), we followed up the 12,094 live births who belonged to the Kaiser Permanente Medical Care Plan between 1981 and 1997. Maternal serum samples were obtained during pregnancy in nearly all gravidas, frozen immediately, and archived at –20°C.
All subjects provided written informed consent for human investigation. The protocol was approved by the institutional review boards of the New York State Psychiatric Institute and the Kaiser Foundation Research Institute. Subjects with potential schizophrenia spectrum disorders (N=183) were screened by linking the Child Health and Development Study and the Kaiser Permanente Medical Care Plan registries for psychiatric treatment for ICD-9 295–299 diagnoses or antipsychotic use. The 170 living potential schizophrenia spectrum disorder cases (13 were deceased) were targeted for face-to-face interviews with the Diagnostic Interview for Genetic Studies
(7), which was administered to 107 targeted subjects for DSM-IV diagnosis. Noninterviewed potential cases (N=76) were diagnosed by chart review. Schizophrenia spectrum disorders were defined as schizophrenia, schizoaffective disorder, schizotypal personality disorder, delusional disorder, and other schizophrenia spectrum psychosis. The protocol yielded 71 subjects with schizophrenia spectrum disorders; 85% had either schizophrenia or schizoaffective disorder
(6) .
Among the 71 patients, 60 had at least one available prenatal serum sample. The last serum sample from each pregnancy (generally third trimester or perinatal) was used. Comparison subjects (N=110) were selected following exclusion of siblings of cases, subjects with schizophrenia spectrum disorders and major affective disorders, and subjects without prenatal sera. They were additionally matched on date of birth, length of cohort membership, sex, and gestational timing of sera
(6) .
Herpes simplex virus type 1 and type 2 IgG were quantified by using the HerpesSelect enzyme-linked immunosorbent assays (ELISA) (Focus Technologies, Cypress, Calif.), and cytomegalovirus IgG was quantified with an ELISA kit from Wampole Laboratories, Princeton, N.J. Sensitivity ranged from 96% to 100%, and specificity ranged from 95% to 96%.
Point and interval estimates of odds ratios were obtained by fitting conditional logistic regression models for matched sets
(8) . We tested whether maternal IgG seropositivity to herpes simplex virus type 2 was associated with the risk of schizophrenia spectrum disorders. In accordance with standard practice, IgG optical density values greater than the cutoff optical density (index values >1.0) were seropositive, optical density values 0.9–1.0 were “equivocal,” and optical density values <0.9 were seronegative. We also tested the relationship between maternal herpes simplex virus type 2 IgG antibody levels and the risk of schizophrenia spectrum disorders, restricting the analyses to herpes simplex virus type 2 seropositive mothers (defined as optical density values >1), based on previous precedents in the literature
(9) .
Among potential confounders (maternal age, ethnicity, education, age and sex of offspring, and gestational age of sera), only maternal ethnicity was associated with herpes simplex virus type 2 (χ 2 =10.84, df=2, p=0.004). We also adjusted for maternal education given previous associations between herpes simplex virus type 2 and lower social class.
Results
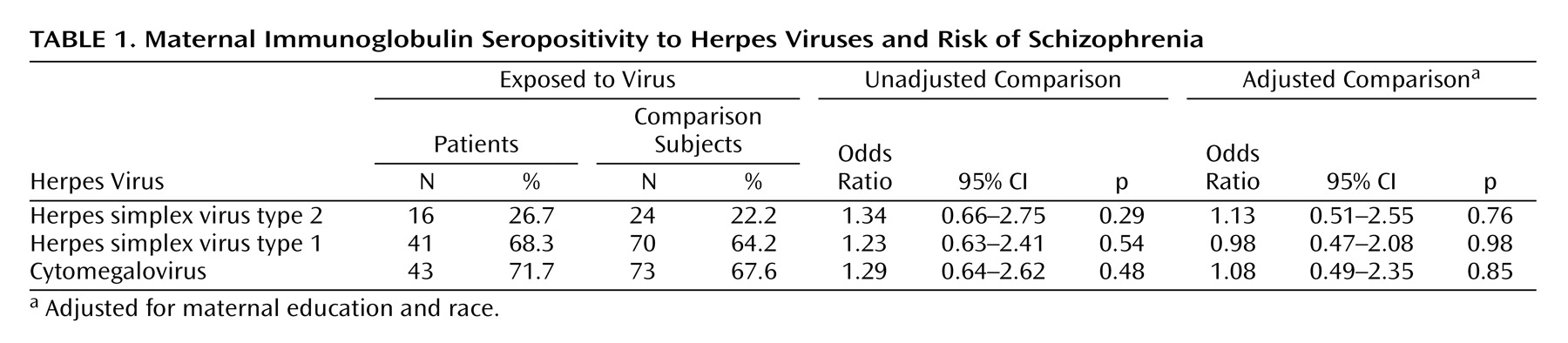
There was no association between maternal IgG seropositivity to herpes simplex virus type 2 and the risk of schizophrenia spectrum disorders (
Table 1 ). There was also no association between maternal IgG antibody to herpes simplex virus type 2 and the risk of schizophrenia spectrum disorders among subjects who were seropositive to herpes simplex virus type 2; the adjusted odds ratio was 0.82 (95% confidence interval [CI]=0.09–7.75, p=0.86). In accordance with Buka et al.
(5), we reran the analysis of maternal herpes simplex virus type 2 IgG for all subjects regardless of seropositivity status. No association between maternal IgG level to herpes simplex virus type 2 and schizophrenia spectrum disorders was found (adjusted odds ratio=1.04, 95% CI=0.76–1.43, p=0.79). In exploratory analyses, we found no association between schizophrenia spectrum disorders and seropositivity to maternal herpes simplex virus type 1 or cytomegalovirus (
Table 1 ) or between schizophrenia spectrum disorders and maternal herpes simplex virus type 1 IgG (adjusted odds ratio=1.37, 95% CI=0.63–2.97, p=0.43) and cytomegalovirus IgG (adjusted odds ratio=0.17, 95% CI=0.02–1.63, p=0.12) in seropositive subjects.
Discussion
We found no evidence to implicate maternal IgG seropositivity to herpes simplex virus type 2 or to maternal IgG herpes simplex virus type 2 antibody levels in the risk of schizophrenia among adult offspring. The study strengths included prospective assessment of exposure, use of direct biomarkers for herpes simplex virus type 2 antibody, a well-characterized birth cohort, diagnosis by direct interviews, and a representative sample of matched comparison subjects.
Our result for herpes simplex virus type 2 differed from that of Buka et al.
(5), who demonstrated an association between maternal herpes simplex virus type 2 IgG antibody levels and schizophrenia in offspring.
These differences might be explained by the fact that, unlike the previous study, our patient sample was confined to those with schizophrenia spectrum disorders, rather than broadly defined psychosis. In addition, the larger number of patients in our study may have guarded against chance fluctuations in the results. One further difference is that in the previous study, all subjects (including those who were seronegative) were apparently included in the analyses of herpes simplex virus type 2 IgG antibody levels. Nonetheless, when we used the same method for classifying exposure status as in that study, there was no association between maternal herpes simplex virus type 2 IgG antibody levels and schizophrenia.
In an earlier report on this birth cohort, we demonstrated an association between influenza during the first half of pregnancy and the risk of schizophrenia spectrum disorders
(2) . The negative findings of the present study persisted after adjustment for prenatal influenza exposure. The findings were also negative whether the outcome was defined as schizophrenia only or other schizophrenia spectrum disorders. Classification of the equivocal results as positive or negative also had no impact on the findings (all results are available upon request from the first author).
There are three limitations worth noting. First, the present study was not designed to rule out maternal exposure to active or reactivated infection as a risk factor for schizophrenia because IgG antibody can only definitively document a previous history of exposure. Second, although the sample size was modest, it was more than twice the size of the study by Buka et al.
(5), and the odds ratios in our study were all close to 1. Third, although the study used sera that had been frozen for more than 30 years, IgG antibody is very stable in stored frozen sera.
To conclude, in a large birth cohort study, we found no association between maternal IgG seropositivity or IgG antibody levels to herpes simplex virus type 2 and risk of schizophrenia. Our findings do not support a previous report of such a relationship.